1 Introduction
Immunotherapy has revolutionized most cancers therapy and rejuvenated the sector of tumor immunology by enhancing affected person’s immune response (1–3). Most cancers immunotherapy gives unprecedented charges of sturdy medical advantages for sufferers with several types of most cancers, however the identification of potential responders earlier than immunotherapy stays to be a problem (4).
Tertiary lymphoid constructions (TLSs), generally known as ectopic lymphoid constructions, are organized aggregates of immune cells resembling secondary lymphoid organs (SLO, e.g. lymph nodes and spleen). Completely different from SLO, TLSs come up postnatally in nonlymphoid tissues beneath chronically infected environments, reminiscent of autoimmune ailments, allograft rejection, continual irritation and most cancers (5). Though the mature TLSs have related construction to SLO, the mobile make-up and molecular pathways concerned within the technique of TLS formation differ attributable to completely different native tissue context and illness. The presence of TLSs sometimes contributed to superior prognosis of most cancers sufferers (6). Furthermore, it has been reported that the presence of TLSs in tumor might predict an improved outcomes in most cancers sufferers handled with immune checkpoint inhibitors (ICI) independently of PD-L1 standing (7, 8). For instance, the survival price of TLS constructive sufferers has been proved to be greater than that of TLS damaging group in non-small-cell lung most cancers (NSCLC) sufferers handled with ICI (9). Nonetheless, not all TLSs positively contribute to the immune response in opposition to most cancers, which can be attributed to distinction in TLS density, location or maturation standing.
Subsequently, this overview goals to explain the traits of TLSs, together with mobile composition, density, location, maturity and gene signature, and supply new perception into the predictive worth for immunotherapy response in cancers.
2 Characterization of TLSs
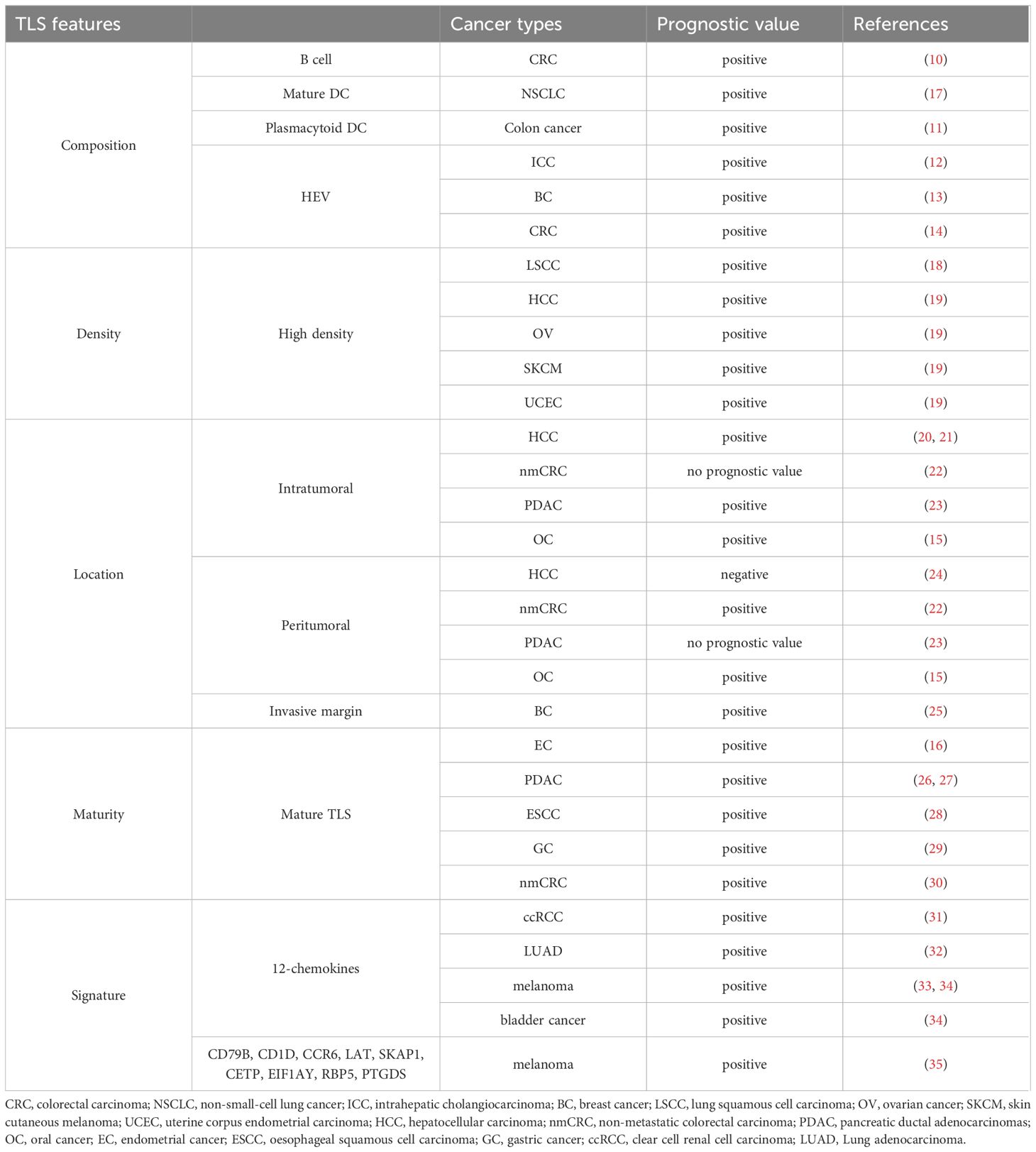
The principle traits of TLSs embody composition, density, location, maturation standing, and signature (Determine 1). We summarized the findings in regards to the efficiency of those TLS options in most cancers affected person prognosis (10–16) (Desk 1).
Determine 1 Mannequin of TLS traits. TLS traits embody composition, density, location, maturation standing and signature.
2.1 TLS composition
TLSs primarily encompass CD3+T-cell-rich area, CD20+B-cell-rich area, plasma cells (PCs), dendritic cells (DCs), and fibroblastic reticular cells (FRCs) (36, 37). Cytotoxic granule- expressing CD8+ T cells have been detected in surrounding T cell zone, as have CD4+ T cells oriented in the direction of a Th1 cell phenotype and CD4+ regulatory T (Treg) cells (38). The interior B cell zone additionally contains CD21+ follicular dendritic cells (FDCs), germinal heart (GC), CD83+ mature DCs, that are markers of mature TLSs (5). The neighborhood of TLSs consists of lymphatic vessels, which specific podoplanin and produce CC-chemokine ligand 21 (CCL21), and excessive endothelial venules (HEVs), that are characterised by the markers of peripheral node addressin (PNAd) (38). The follicles can additional comprise scattered CD68+ macrophages for clearance of apoptotic cells (5).
TLS formation might be induced by tissue-specific expression of chemokines. Heterogeneity of those driving elements could result in variations in TLS elements. Intriguingly, some particular cell varieties have been noticed within the TLSs beneath sure situations. LAMP+ DCs (mature DCs) have been thought of to be dependable marker of TLSs in NSCLC as they have been nearly completely present in these constructions (39). The density of LAMP+ DCs has been proved to be correlated with favorable medical outcomes in NSCLC (17). A qualitative shift within the group of TLSs has been present in Helicobacter hepaticum (Hhep) colonized mice in colorectal most cancers (CRC), during which an elevated presence of CD11c+ cells was discovered within the T cell zone of TLSs, according to a rise in DCs (40). A novel CD20+CD22+ADAM28+ B-cell subpopulation inside TLSs has been reported to be current in ICI responders. These cells have been named as ICI‐Responsive B cells (BIR) and have been additional recognized as a subset of reminiscence B cells that promoted the response to ICI remedy (41).
2.2 TLS density
TLS density varies amongst completely different people, even for a given most cancers kind and stage of the illness, emphasizing that particular person tumor microenvironment (TME) may be kind of permissive to lymphoid neogenesis (42). Excessive TLS density in tumors typically correlates with higher prognosis. It has been reported that the expression of transcription elements associated to adaptive immunity was considerably upregulated in TLS-high tumors (18). Sufferers with excessive TLS density exhibited considerably improved total survival (OS) in hepatocellular carcinoma (HCC), ovarian most cancers (OV), pores and skin cutaneous melanoma (SKCM) and uterine corpus endometrial carcinoma (UCEC) (19, 43). In a lung squamous cell most cancers (LSCC) cohort, excessive TLS density considerably correlated with improved progression-free survival (PFS) (16). Other than main tumors, a better degree of TLSs in metastatic tumors additionally confirmed considerably higher OS (44–47).
2.3 TLS location
TLSs may be discovered within the peritumor, invasive margin and heart of tumors (27). The placement of TLSs could also be vital in predicting outcomes, related to its perform within the tumor immune response (48).
Some research confirmed that sufferers with excessive degree of peritumoral TLSs exhibited worse disease-free survival (DFS) and OS in breast most cancers, cholangiocarcinoma (CCA), HCC and colorectal most cancers liver metastases (CRLM) (24, 25, 49, 50). Zhang et al. discovered that the frequency of CD4+Bcl6+ T follicular helper (Tfh) cells was considerably elevated in intratumoral TLSs in comparison with peritumoral TLSs in CRLM (50). Nonetheless, one examine discovered peritumoral TLSs as an impartial and favorable prognostic consider each OS and DFS for non-metastatic colorectal carcinoma (nmCRC) sufferers (22).
Not like the twin position of peritumoral TLSs in prognosis analysis, intratumoral TLSs have been proved to be related to higher consequence for most cancers sufferers, together with HCC and pancreatic most cancers (20, 21, 23). Tumor tissues with intratumoral TLSs confirmed considerably greater infiltration of T and B cells and decrease infiltration of immunosuppressive cells (23). TLSs on the invasive margin have additionally been validated as an vital constructive predictor of affected person outcomes (48, 51).
2.4 TLS maturity
In keeping with the mobile compositions, TLSs may be labeled as follows: 1) Early TLSs (E-TLSs), distinguished by lymphocytic aggregates that lack a DC scaffold and vascularization; 2) Main follicle-like TLSs (PFL-TLSs), often known as immature TLSs, comprised of T-cell and B-cell zones with FDCs however no GC; 3) Secondary follicle-like TLSs (SFL-TLSs), often known as mature TLSs, comprised of lymphatic vessels, remoted T-cell zones and a B-cell follicle with GC (30). Quite a few research have demonstrated that plasma cells, CD8+ T cells, and CD4+ T cells have been extra enriched in PFL-TLSs and SFL-TLSs (22, 28, 52).
Mature TLSs have been reported to enhance the prognosis of oesophageal squaenmous cell carcinoma (ESCC) and gastric most cancers sufferers (28, 29). The cumulative threat of recurrence was considerably greater in sufferers with low SFL-TLSs in nmCRC (30). Mature TLSs supported antitumor adaptive immunity in pancreatic ductal adenocarcinomas (PDAC) (26, 27) and their formation was related to a greater prognosis in laryngeal squamous cell carcinoma (LSCC) with immunotherapy (52). Thus, mature TLSs have been confirmed to positively correlate with the prognosis and immunotherapy response of most cancers sufferers.
2.5 TLS signature
Other than H&E staining and immunohistochemistry (IHC) with multiplex chosen markers to detect TLSs, transcriptomic analyses have additionally been developed to find out TLS-associated gene signatures in recent times (53). A 12-chemokine signature (together with CCL2, CCL3, CCL4, CCL5, CCL8, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11, and CXCL13) is probably the most extensively used signature for the quantification of TLSs in a number of strong tumors, together with CRC, melanoma, HCC and breast most cancers, and many others (19, 33). Research discovered that sufferers with excessive TLS signature displayed a greater survival than these with low TLS signature, displaying a marked affiliation between TLS signature and the survival of most cancers sufferers (32). Li et al. validated the medical utility of the 12-chemokine TLS signature for predicting immunotherapy response through the use of two publicly out there datasets. A excessive TLS signature rating indicated robust immune infiltration and immune responses in each datasets (34). One other examine by Xu et al. established TLS clusters in clear cell renal cell carcinoma (ccRCC) utilizing machine studying algorithms and the 12-chemokine gene signature. Distinct variations have been noticed in survival, immune cell distribution, immunotherapy response among the many TLS clusters (31). Cabrita et al. developed a gene signature (CD79B, CD1D, CCR6, LAT, SKAP1, CETP, EIF1AY, RBP5, and PTGDS) related to TLSs in melanoma sufferers, which predicted medical outcomes of melanoma sufferers handled with ICI (35).
3 Inducing elements of TLS formation
Tumor particular lymphocytes and stromal cells supply chemokines or cytokines required for TLS formation. Chemokines and lymphotoxins (LTs) are important for the clustering of B/T cells and the event of lymphoid constructions throughout TLS neogenesis. For instance, CXCL13 and CXCL12 promoted the recruitment of B cells (54); CCL21 induced LTs expression on naive CD4 T cells and induced extra organized infiltrates (55). On this part, we summarized the principle mobile inducers of TLSs and potential pharmaceutical manners to induce TLS formation (Determine 2).
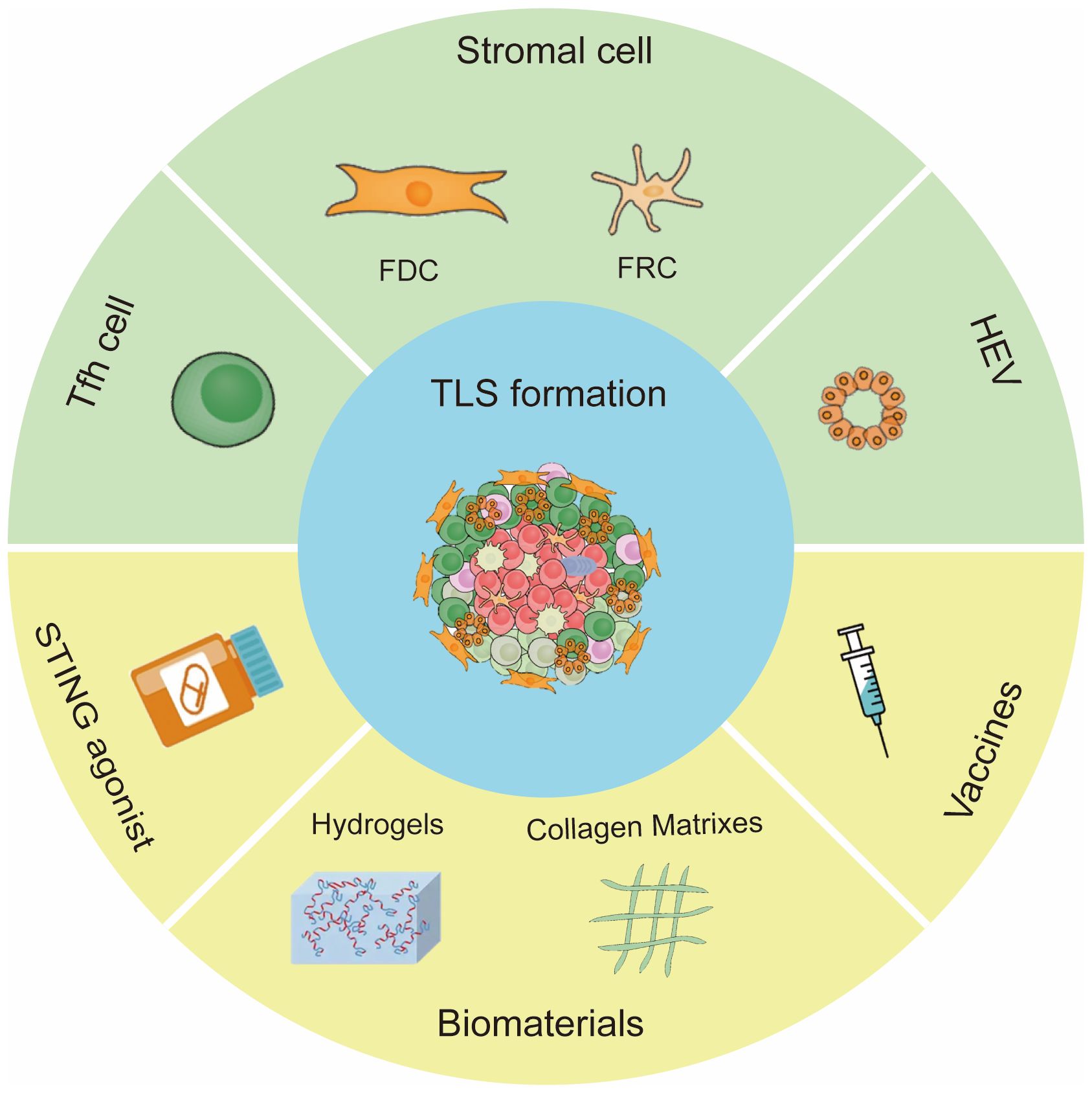
Determine 2 Inducing elements of TLS formation. The principle cells for TLS formation are CXCL13 -expressing Tfh cells, stromal cells and HEVs. Strategies to induce TLS formation embody STING agonist, vaccine and biomaterials.
3.1 Mobile inducers of native TLSs
3.1.1 CXCL13-producing T follicular helper cell
Tfh cells, a subset of CD4+ T helper cells, are specialised in serving to B cell proliferation, survival, and differentiation, thus supporting antibody manufacturing and reminiscence formation. The CXCL13-expressing Tfh cells are generally co-localized with B cells within the tissue, permitting for Tfh cell to assist the perform of B cells and related to the formation of TLSs (49, 56). Notably, enhanced technology of Tfh cells created a chemokine area of interest that promoted spontaneous meeting of TLSs at tumor beds (57). A latest examine implied that CXCL13-producing CD4+ T cells have been concerned within the early stage of TLS formation (58), which according to one other examine indicating that TLS formation was depending on CXCL13 signaling, doubtlessly attributable to its impact on the recruitment of CXCR5+ B cells (59).
3.1.2 Stromal cell
Stromal cells can differentiate into lymphoid tissue organizer (LTo) cells following stimulation by the lymphotoxin (LT) β receptor. LTo cells specific chemokines and cell adhesion molecules that recruit and manage the B and T cell areas of the organ. LTo cells endure additional differentiation into the stromal cell subsets current in grownup lymph nodes (LN) reminiscent of FRCs of the T zone, FDCs current in B cell follicles and GCs (60). Throughout the tumor microenvironment, the native cross-talk between immune cells and stromal components results in the manufacturing of a sequence of pro-inflammatory cytokines and TNF receptor household elements that decide the formation of TLSs (51).
Zhu et al. created a mouse mannequin of TLSs by implanting LN-derived stromal cells that specific markers of FRCs. They discovered that TLSs have been shaped by growth of stromal cells and gradual infiltration of B cells, CD4+ and CD8+ T cells (61). Nayar et al. demonstrated that fibroblasts might drive TLS formation in a melanoma mannequin whereby they developed traits of LTo cells in response to TNF-receptor sign (62). Others discovered a CXCL13-CXCR5 chemotactic axis supported the proliferation of most cancers related fibroblasts (CAF) with LTo traits and TLS improvement (63, 64).
3.1.3 HEV
HEVs specific excessive ranges of ligands for lymphocyte adhesion molecules, which facilitate the trafficking of T and B cells to the native tissue microenvironment. Tumor HEVs enhance intratumoral lymphocytes infiltration, facilitating TLS maturation (65). Wang et al. induced endothelial differentiation from pluripotent stem cells after which constructed HEV-like organoids (HEVO). Upon transplantation into mice, HEVO promoted useful TLS formation by recruiting lymphocytes and enhanced antitumor exercise (66).
3.2 Manners of TLS induction
3.2.1 STING agonist
A number of stimulators of interferon gene (STING) agonists have been developed for most cancers remedy and have achieved promising ends in pre-clinical work (67). Therapy with low-dose STING agonist ADU-S100 slowed tumor development and promoted the formation of non-classical TLSs in murine B16 melanomas (68). Activation of STING throughout the TME elevated the manufacturing of antiangiogenic elements and TLS-inducing chemokines and cytokines, leading to improved vascular normalization (VN), enhanced tumor infiltration of CD11c+ DCs and CD8+ T cells and native TLS neogenesis (69, 70).
3.2.2 Vaccines
Vaccines can stimulate physique’s innate immunity and powerful T cell response to fight infectious ailments and cancers. Lutz et al. first reported that granulocyte-macrophage colony-stimulating issue (GM-CSF)–secreting, allogeneic PDAC vaccine (GVAX) induced TLS formation and extra T-cell infiltration in PDAC sufferers (71). Maldonado et al. noticed reminiscence T cells infiltration and TLS formation in vaccinated topics with high-grade cervical intraepithelial neoplasias (72). TLS formation was additionally noticed in resected stage IIB-IV melanoma after vaccination with AS15 and IFA (two most cancers vaccines) (73).
Rising of nanovaccines has improved focused supply, extended circulation and antigen presentation (74). Wen et al. demonstrated that the nanovaccine consisting of Epstein-Barr virus nuclear antigen 1 (EBNA1) and a bi-adjuvant of Mn2+ and cytosine-phosphate-guanine (CpG) formulated with tannic acid can foster TLS formation. The nanovaccine activated LT-α/β pathways, subsequently enhancing the expression of downstream chemokines CCL19/CCL21, CXCL10 and CXCL13 within the TME (75). Not too long ago, researchers developed antigen-clustered nanovaccine (ACNVax) to activate immune cell. ACNVax plus anti-PD-1 antibody stimulated TLS formation and achieved long-term antitumor efficacy (76).
3.2.3 Biomaterials
Current research revealed that some biomaterials not solely function drug carriers, but in addition have intrinsic immunoregulatory results (77). Collagens are main elements of the extracellular matrix of connective tissues (78). Suematsu et al. initially used a collagen sponge biomatrix embedded with LTα-expressing stromal cells and transplanted in vivo to induce constructions just like TLSs (79). Subsequently, TLSs have been additionally efficiently induced utilizing collagen sponge scaffolds comprise chemokines and soluble RANK ligand (80).
Hydrogels are three-dimensional crosslinked polymer meshwork. With its properties, hydrogels can lengthen residence time as drug vector and are appropriate for biomedical functions (81). Hydrogel preparations with BAFF-producing stromal cells and IL-4 promoted GC-like response in B cell and antibody class switching in vitro (82, 83). One other examine described that STING-activating hydrogel (ZCCG) facilitated the formation of TLSs by recruiting immune cells and enhanced antitumor immunity (84).
4 The position of TLSs in predicting immunotherapy response
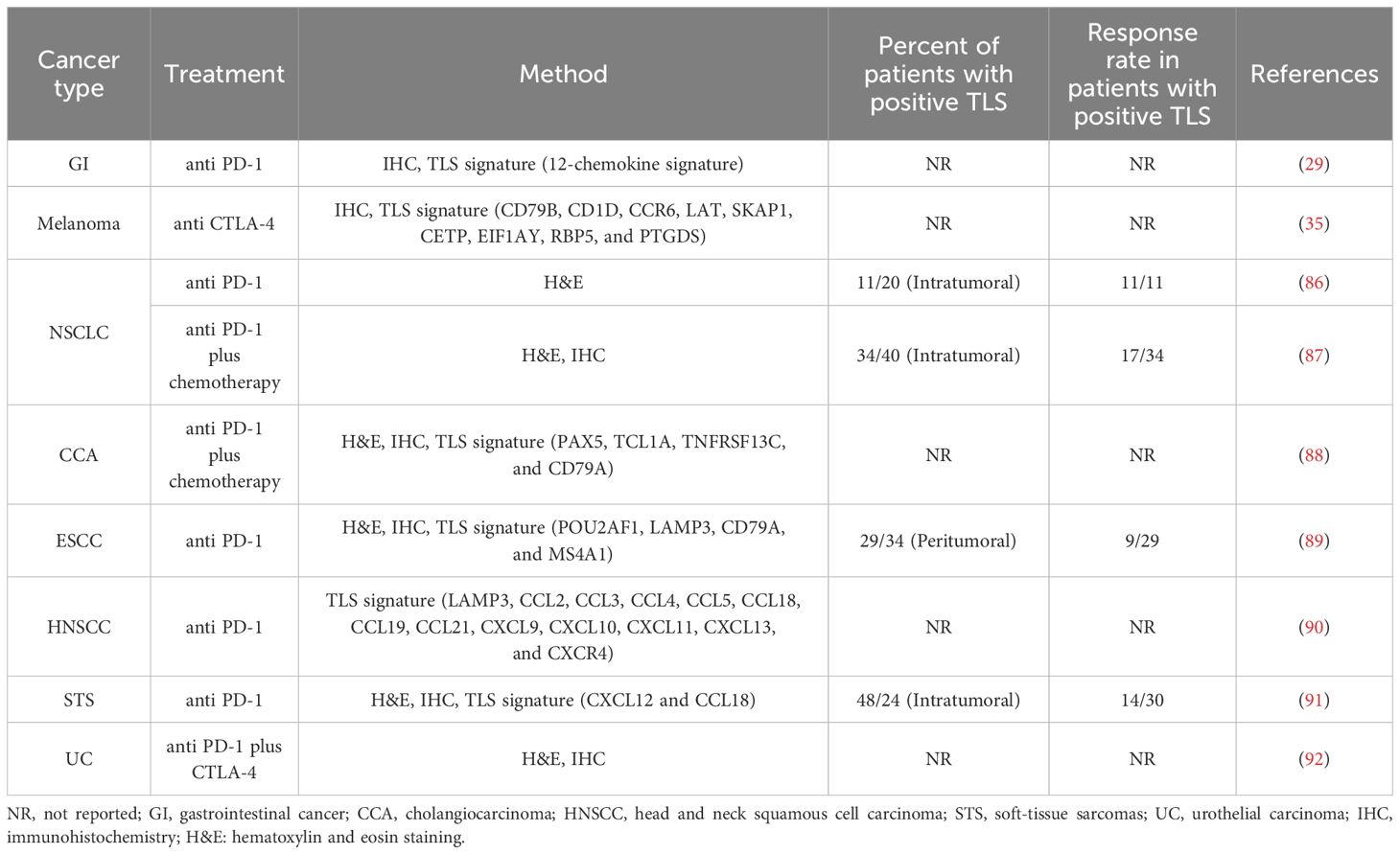
ICI performs an vital position in most cancers immunotherapy, and sustains a long-term immune response for most cancers sufferers. The 2 main ICI approaches are anti-PD1/PD-L1 and anti-CTLA-4 antibodies (85). ICI therapy induced the growth of CD8+ T cells which was not noticed earlier than therapy, indicating that ICI response was pushed by incoming T cells. TLSs could also be instrumental to restart antitumor protection throughout ICI therapy by mounting a contemporary adaptive immune response utilizing incoming B and T cells (36). Research have demonstrated that TLSs might function potential biomarker in predicting response to ICI remedy (86) (Desk 2).
4.1 Melanoma
Immunotherapy has turn into an vital a part of the therapy for sufferers with superior melanoma, however its medical efficacy varies amongst sufferers. The vast majority of sufferers handled with ICI didn’t present important medical advantages (93). Griss et al. demonstrated that the frequency of plasmablast-like B cells in pre-therapy melanomas predicted response and survival to immune checkpoint blockade. Anti-CD20 therapy brought on a downregulation of tumor-induced plasmablast-like B cells together with a major discount in TLSs in metastatic melanoma (94). Ding et al. confirmed that melanoma affected person who responded to ICI had elevated variety of GC B cells and its related Tfh cells, indicative of TLS formation, and had considerably longer OS than these with none (95). One other examine by Alexandra et al. gathered a set of tumor biopsies from melanoma sufferers who have been receiving CTLA-4 blockade. Trichotomizing gene-expression knowledge on the premise of the TLS signature revealed that TLS-high tumors have been related to considerably elevated survival after CTLA-4 blockade (35).
4.2 NSCLC
With the invention of immunotherapy, the therapeutic paradigm for sufferers with superior lung most cancers has essentially reworked (96). The revolution in immunotherapy, particularly the event of ICIs, has dramatically altered the NSCLC therapy panorama (97). Brunet et al. confirmed that the presence of TLSs was not solely a prognostic marker in superior phases of NSCLC, but in addition a selected biomarker predictive of response to ICIs (98). Likewise, Patil et al. demonstrated that plasma-cell-rich tumors could portend OS profit in NSCLC sufferers handled with ICI. The plasma cell signature was enriched in tumors with TLSs and/or lymphoid aggregates. Sufferers with TLS constructive tumors exhibited improved OS with PD-L1 blockade (atezolizumab) (9). Wu et al. discovered that overexpression of most genes in TLS signature indicated an excellent prognosis in sufferers with NSCLC receiving ICI remedy (41).
4.3 Gastrointestinal (GI) cancers
GI cancers embody esophageal, gastric, liver, biliary system, pancreatic, and colorectal most cancers. A meta-analysis together with 32 research demonstrated that TLSs have been important predictor of the prognosis of GI most cancers and have the potential to turn into biomarker for immunotherapy responses in GI most cancers sufferers (99). Jiang et al. discovered that greater TLS rating was correlated with a superior response to PD1 blockade remedy in sufferers with gastric most cancers, indicating that the TLS rating could be a brand new predictor for PD1 inhibitor remedy response (29). Shang et al. analyzed a cohort of 100 CCA sufferers who acquired first-line chemotherapy mixed with ICIs to forestall postoperative recurrence. Additional knowledge indicated {that a} excessive density of intratumoral TLSs in pre-treatment tumor tissues predicted a greater prognosis in sufferers with immunotherapy and the presence of intratumoral TLSs have been related to a chronic OS and PFS. The examine established a four-gene TLS signature as practicable biomarker for TLS identification and demonstrated that the spatial distribution and abundance of TLSs profoundly affected the prognosis and the immunotherapy response in CCA (88). Kinker et al. confirmed that the expression of a gene signature reflecting mature TLSs have been enriched in pretreatment biopsies from PDAC sufferers with longer survival after receiving completely different chemoimmunotherapy regimens (26). Hayashi et al. derived a 4-gene TLS signature comprised of POU2AF1, LAMP3, CD79A and MS4A1 and located a considerably greater expression of this 4-gene TLS signature in responders to anti-PD-1 remedy as in comparison with non-responders in ESCC (89). Küçükköse et al. established a sequence of patient-derived organoids (PDOs) from MSI-H mCRC tumors to generate spontaneous metastasis fashions in mice with or with out a human immune system (HIS). HIS mice with PDO-initiated MSI-H mCRC have been then used to mannequin ICI remedy. Anti-PD-1 and anti-CTLA-4 strongly lowered the expansion of main tumors and liver metastases, however peritoneal metastases have been refractory to ICI. B cell inflow and TLS formation have been noticed in ICI-responding main tumors and liver metastases, whereas ICI-refractory peritoneal metastases have been devoid of B cells and TLSs (100).
4.4 Different cancers
Some research reported an upregulation of genes encoding chemokines concerned in TLS formation within the responder sufferers of HNSCC, bladder most cancers or soft-tissue sarcomas (STS) handled with ICIs. These excessive TLS signatures have been related to higher OS (90, 91, 101). Meylan et al. evaluated the B cell responses inside TLSs in RCC, and located IgG- and IgA-producing plasma cells infiltated into the TLS-positive tumors. RCC sufferers with excessive IgG-stained tumor cells had longer PFS and better immunotherapy response charges (102).
5 The position of TLSs in evaluating neoadjuvant immunotherapy efficacy
Neoadjuvant immunotherapy is assumed to supply long-term remissions by induction of immune responses and has entered normal of care in NSCLC and melanoma (103–105). Many research have used TLSs as marker to guage neoadjuvant immunotherapy efficacy. Wang et al. reported that TLS‐constructive TNBC sufferers achieved a substantial response after neoadjuvant immunotherapy with six cycles of camrelizumab. The neoadjuvant immunotherapy impact was not evident in TLS-negative affected person (106). One other examine by Solar et al. confirmed that TLS abundance and maturity have been greater within the neoadjuvant chemoimmunotherapy group than in neoadjuvant chemotherapy group and therapy naive group in NSCLC. Sufferers with main pathological response (MPR) had extra mature TLSs than these with non-MPR in each neoadjuvant chemoimmunotherapy and neoadjuvant chemotherapy group (87). Helmink et al. assessed the density and distribution of B cells in addition to their relationship to TLSs in melanoma and RCC sufferers handled with neoadjuvant ICI. The density of CD20+ B cells and TLSs have been greater in responders than in non-responders in neoadjuvant melanoma cohort, significantly in early on-treatment samples (107). Gao et al. reported the primary pilot mixture neoadjuvant trial with anti-PD-L1 (durvalumab) plus anti-CTLA-4 (tremelimumab) in cisplatin-ineligible urothelial carcinoma sufferers. They noticed a better density of TLSs in pre-treatment tumor tissues of responder sufferers as in comparison with non-responder affected person. Larger density of TLSs in pre-treatment tumor tissues was correlated with longer OS (92).
6 Dialogue
TLSs can function enticing biomarker for the prediction of immunotherapy response in opposition to most cancers. Nonetheless, heterogeneity of TLS elements induced by tissue-specific elements could result in variations in immune responses. As well as, the buildup of regulatory immune cells inside TLSs could dampen immune responses, resulting in the inactivation of TLSs. Subsequently, a complete analysis of TLS options would supply extra correct prediction efficacy.
Many research have described the position of TLSs in most cancers immunotherapy, however the mechanisms underlying the formation of TLSs in immunotherapy stay unclear. Rodriguez et al. confirmed that immunotherapy can improve TLS quantity and measurement in mouse fashions (63). Some research explored the mixture of most cancers immunotherapy with vaccines or biomaterials to induce TLSs, most likely by a mechanism involving a community of cells and chemokines (75, 108). Current advances demonstrated that synthetic or inducible TLSs (iTLSs) maintain nice promise to enhance medical outcomes post-immunotherapy. Along with STING angonist, vaccine and biomaterials, iTLSs may also be achieved utilizing stromal vascular fraction (109) or cell-free constructs (110). In addition to, oncolytic virotherapies have the potential to change chilly tumors to sizzling tumors, and due to this fact might be good drivers of TLS neogenesis (111). It appears extremely fascinating to induce and/or increase TLS improvement as new facet of most cancers immunotherapy. Nonetheless, the heterogeneity of TLSs in several tumors and people, in addition to the issue in number of supplies for inducing TLSs nonetheless make it a problem for iTLSs to be really utilized in medical observe.
Unraveling the interaction between antitumor response and autoimmunity mediated by T cells, B cells and autoantibodies throughout TLS induction is crucial. Antitumoral immunity and autoimmune response are related in most cancers sufferers. A examine has reported that TLSs have been enriched in OMAS (an autoimmune illness) related neuroblastomas (112). This affiliation could also be attributable to that an environment friendly TLS-induced antitumor response throughout the TME results in tumor cell demise and subsequent launch of huge antigens that may activate autoreactive T and B cells.
In conclusion, this overview revealed that the presence of TLSs indicated energetic antitumor immune responses and useful outcomes for most cancers sufferers. The induction remedy of TLSs could present new alternatives to enhance the present immunotherapeutic therapies.
Creator contributions
CC: Writing – overview & enhancing. XC: Writing – overview & enhancing. MW: Writing – overview & enhancing. FY: Writing – unique draft. JY: Writing – unique draft.
Funding
The creator(s) declare monetary help was acquired for the analysis, authorship, and/or publication of this text. This work was supported by the Nationwide Pure Science Basis of China (grant no. 82072725 to XC, no. 81972333 to CC and no. 82303406 to MW).
Acknowledgments
We want to thank CC and MW for revising the language of this paper.
Battle of curiosity
The authors declare that the analysis was performed within the absence of any industrial or monetary relationships that might be construed as a possible battle of curiosity.
Writer’s be aware
All claims expressed on this article are solely these of the authors and don’t essentially characterize these of their affiliated organizations, or these of the writer, the editors and the reviewers. Any product which may be evaluated on this article, or declare which may be made by its producer, will not be assured or endorsed by the writer.
References
1. Zhang Y, Zhang Z. The historical past and advances in most cancers immunotherapy: understanding the traits of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
PubMed Summary | CrossRef Full Textual content | Google Scholar
2. Helmy KY, Patel SA, Nahas GR, Rameshwar P. Most cancers immunotherapy: accomplishments up to now and future promise. Ther Supply. (2013) 4:1307–20. doi: 10.4155/tde.13.88
4. Stroll EE, Yohe SL, Beckman A, SChade A, Zutter MM, Pfeifer J, et al. The most cancers immunotherapy biomarker testing panorama. Arch Pathol Lab Med. (2020) 144:706–24. doi: 10.5858/arpa.2018-0584-CP
PubMed Summary | CrossRef Full Textual content | Google Scholar
5. Schumacher TN, Thommen DS. Tertiary lymphoid constructions in most cancers. Sci (New York NY). (2022) 375:eabf9419. doi: 10.1126/science.abf9419
6. Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid constructions in most cancers and past. Traits Immunol. (2014) 35:571–80. doi: 10.1016/j.it.2014.09.006
PubMed Summary | CrossRef Full Textual content | Google Scholar
7. Vanhersecke L, Brunet M, Guégan JP, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid constructions predict immune checkpoint inhibitor efficacy in strong tumors independently of PD-L1 expression. Nat Most cancers. (2021) 2:794–802. doi: 10.1038/s43018-021-00232-6
PubMed Summary | CrossRef Full Textual content | Google Scholar
8. Lee JY, Kannan B, Lim BY, Li Z, Lim AH, Loh JW, et al. The multi-dimensional biomarker panorama in most cancers immunotherapy. Int J Mol Sci. (2022) 23:7839. doi: 10.3390/ijms23147839
9. Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung most cancers. Most cancers Cell. (2022) 40:289–300.e4. doi: 10.1016/j.ccell.2022.02.002
PubMed Summary | CrossRef Full Textual content | Google Scholar
10. Xia J, Xie Z, Niu G, Lu Z, Wang Z, Xing Y, et al. Single-cell panorama and medical outcomes of infiltrating B cells in colorectal most cancers. Immunology. (2023) 168:135–51. doi: 10.1111/imm.13568
PubMed Summary | CrossRef Full Textual content | Google Scholar
11. Kießler M, Plesca I, Sommer U, Wehner R, Wilczkowski F, Müller L, et al. Tumor-infiltrating plasmacytoid dendritic cells are related to survival in human colon most cancers. J Immunother Most cancers. (2021) 9:e001813. doi: 10.1136/jitc-2020-001813
PubMed Summary | CrossRef Full Textual content | Google Scholar
12. Zhou Y, Gu Q, Zhu L, Zhang S, Wu H, Pu X, et al. Excessive endothelial venule is a prognostic immune-related biomarker in sufferers with resected intrahepatic cholangiocarcinoma. Cell Proliferation. (2023) 56:e13513. doi: 10.1111/cpr.13513
PubMed Summary | CrossRef Full Textual content | Google Scholar
13. Sawada J, Hiraoka N, Qi R, Jiang L, Fournier-Goss AE, Yoshida M, et al. Molecular signature of tumor-associated excessive endothelial venules that may predict breast most cancers survival. Most cancers Immunol Res. (2022) 10:468–81. doi: 10.1158/2326-6066.Cir-21-0369
PubMed Summary | CrossRef Full Textual content | Google Scholar
14. Zhan Z, Shi-Jin L, Yi-Ran Z, Zhi-Lengthy L, Xiao-Xu Z, Hui D, et al. Excessive endothelial venules proportion in tertiary lymphoid construction is a prognostic marker and correlated with anti-tumor immune microenvironment in colorectal most cancers. Ann Med. (2023) 55:114–26. doi: 10.1080/07853890.2022.2153911
PubMed Summary | CrossRef Full Textual content | Google Scholar
15. Li Okay, Guo Q, Zhang X, Dong X, Liu W, Zhang A, et al. Oral cancer-associated tertiary lymphoid constructions: gene expression profile and prognostic worth. Clin Exp Immunol. (2020) 199:172–81. doi: 10.1111/cei.13389
PubMed Summary | CrossRef Full Textual content | Google Scholar
16. Horeweg N, Workel HH, Loiero D, Church DN, Vermij L, Léon-Castillo A, et al. Tertiary lymphoid constructions essential for prognosis in endometrial most cancers sufferers. Nat Commun. (2022) 13:1373. doi: 10.1038/s41467-022-29040-x
PubMed Summary | CrossRef Full Textual content | Google Scholar
17. Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Lengthy-term survival for sufferers with non-small-cell lung most cancers with intratumoral lymphoid constructions. J Clin Oncol: Off J Am Soc Clin Oncol. (2008) 26:4410–7. doi: 10.1200/jco.2007.15.0284
18. Siliņa Okay, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal facilities decide the prognostic relevance of tertiary lymphoid constructions and are impaired by corticosteroids in lung squamous cell carcinoma. Most cancers Res. (2018) 78:1308–20. doi: 10.1158/0008-5472.Can-17-1987
PubMed Summary | CrossRef Full Textual content | Google Scholar
19. Lin Z, Huang L, Li S, Gu J, Cui X, Zhou Y. Pan-cancer evaluation of genomic properties and medical consequence related to tumor tertiary lymphoid construction. Sci Rep. (2020) 10:21530. doi: 10.1038/s41598-020-78560-3
PubMed Summary | CrossRef Full Textual content | Google Scholar
20. Jia W, Yao Q, Wang Y, Mao Z, Zhang T, Li J, et al. Protecting impact of tertiary lymphoid constructions in opposition to hepatocellular carcinoma: New findings from a genetic perspective. Entrance Immunol. (2022) 13:1007426. doi: 10.3389/fimmu.2022.1007426
PubMed Summary | CrossRef Full Textual content | Google Scholar
21. Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid constructions are related to a low threat of early recurrence of hepatocellular carcinoma. J Hepatol. (2019) 70:58–65. doi: 10.1016/j.jhep.2018.09.003
PubMed Summary | CrossRef Full Textual content | Google Scholar
22. Wang Q, Shen X, An R, Bai J, Dong J, Cai H, et al. Peritumoral tertiary lymphoid construction and tumor stroma proportion predict the prognosis of sufferers with non-metastatic colorectal most cancers. Entrance Immunol. (2022) 13:962056. doi: 10.3389/fimmu.2022.962056
PubMed Summary | CrossRef Full Textual content | Google Scholar
23. Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada Okay. Intratumoral tertiary lymphoid organ is a beneficial prognosticator in sufferers with pancreatic most cancers. Br J Most cancers. (2015) 112:1782–90. doi: 10.1038/bjc.2015.145
PubMed Summary | CrossRef Full Textual content | Google Scholar
24. Zhang T, Lei X, Jia W, Li J, Nie Y, Mao Z, et al. Peritumor tertiary lymphoid constructions are related to infiltrating neutrophils and inferior prognosis in hepatocellular carcinoma. Most cancers Med. (2023) 12:3068–78. doi: 10.1002/cam4.5227
PubMed Summary | CrossRef Full Textual content | Google Scholar
25. Sofopoulos M, Fortis SP, Vaxevanis CK, Sotiriadou NN, Arnogiannaki N, Ardavanis A, et al. The prognostic significance of peritumoral tertiary lymphoid constructions in breast most cancers. Most cancers Immunol Immunother. (2019) 68:1733–45. doi: 10.1007/s00262-019-02407-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
26. Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, et al. Mature tertiary lymphoid constructions are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Intestine. (2023) 72:1927–41. doi: 10.1136/gutjnl-2022-328697
PubMed Summary | CrossRef Full Textual content | Google Scholar
27. JG A, Rajamanickam V, Bui C, Bernard B, Pucilowska J, Ballesteros-Merino C, et al. Germinal heart reactions in tertiary lymphoid constructions affiliate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic most cancers. Oncoimmunology. (2021) 10:1900635. doi: 10.1080/2162402x.2021.1900635
PubMed Summary | CrossRef Full Textual content | Google Scholar
28. Ling Y, Zhong J, Weng Z, Lin G, Liu C, Pan C, et al. The prognostic worth and molecular properties of tertiary lymphoid constructions in oesophageal squamous cell carcinoma. Clin Trans Med. (2022) 12:e1074. doi: 10.1002/ctm2.1074
29. Jiang Q, Tian C, Wu H, Min L, Chen H, Chen L, et al. Tertiary lymphoid construction patterns predicted anti-PD1 therapeutic responses in gastric most cancers. Chin J Most cancers Res. (2022) 34:365–82. doi: 10.21147/j.issn.1000-9604.2022.04.05
PubMed Summary | CrossRef Full Textual content | Google Scholar
30. Posch F, Silina Okay, Leibl S, Mündlein A, Moch H, Siebenhüner A, et al. Maturation of tertiary lymphoid constructions and recurrence of stage II and III colorectal most cancers. Oncoimmunology. (2018) 7:e1378844. doi: 10.1080/2162402x.2017.1378844
PubMed Summary | CrossRef Full Textual content | Google Scholar
31. Xu W, Ma C, Liu W, Anwaier A, Tian X, Shi G, et al. Prognostic worth, DNA variation and immunologic options of a tertiary lymphoid structure-related chemokine signature in clear cell renal cell carcinoma. Most cancers Immunol Immunother. (2022) 71:1923–35. doi: 10.1007/s00262-021-03123-y
PubMed Summary | CrossRef Full Textual content | Google Scholar
32. Feng H, Yang F, Qiao L, Zhou Okay, Wang J, Zhang J, et al. Prognostic significance of gene signature of tertiary lymphoid constructions in sufferers with lung adenocarcinoma. Entrance Oncol. (2021) 11:693234. doi: 10.3389/fonc.2021.693234
PubMed Summary | CrossRef Full Textual content | Google Scholar
33. Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like constructions in melanoma: potential for affected person choice for immunotherapy? Sci Rep. (2012) 2:765. doi: 10.1038/srep00765
PubMed Summary | CrossRef Full Textual content | Google Scholar
34. Li X, Wan Z, Liu X, Ou Okay, Yang L. A 12-chemokine gene signature is related to the improved immunogram scores and is related for precision immunotherapy. Med Oncol (Northwood London England). (2022) 39:43. doi: 10.1007/s12032-021-01635-2
35. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid constructions enhance immunotherapy and survival in melanoma. Nature. (2020) 577:561–65. doi: 10.1038/s41586-019-1914-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
36. Lauss M, Donia M, Svane IM, Jönsson G. B cells and tertiary lymphoid constructions: pals or foes in most cancers immunotherapy? Clin Most cancers Res. (2022) 28:1751–58. doi: 10.1158/1078-0432.Ccr-21-1130
PubMed Summary | CrossRef Full Textual content | Google Scholar
38. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid constructions within the period of most cancers immunotherapy. Nat Rev Most cancers. (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
PubMed Summary | CrossRef Full Textual content | Google Scholar
39. Zou J, Zhang Y, Zeng Y, Peng Y, Liu J, Xiao C, et al. Tertiary lymphoid constructions: A possible biomarker for anti-cancer remedy. Cancers (Basel). (2022) 14:5968. doi: 10.3390/cancers14235968
40. Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid constructions and anti-tumor immunity in opposition to colorectal most cancers. Immunity. (2021) 54:2812–24.e4. doi: 10.1016/j.immuni.2021.11.003
PubMed Summary | CrossRef Full Textual content | Google Scholar
41. Wu Z, Zhou J, Xiao Y, Ming J, Zhou J, Dong F, et al. CD20+CD22+ADAM28+ B cells in tertiary lymphoid constructions promote immunotherapy response. Entrance Immunol. (2022) 13:865596. doi: 10.3389/fimmu.2022.865596
PubMed Summary | CrossRef Full Textual content | Google Scholar
42. Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Traits of tertiary lymphoid constructions in main cancers. Oncoimmunology. (2013) 2:e26836. doi: 10.4161/onci.26836
PubMed Summary | CrossRef Full Textual content | Google Scholar
43. Kroeger DR, Milne Okay, Nelson BH. Tumor-infiltrating plasma cells are related to tertiary lymphoid constructions, cytolytic T-cell responses, and superior prognosis in ovarian most cancers. Clin Most cancers Res. (2016) 22:3005–15. doi: 10.1158/1078-0432.Ccr-15-2762
PubMed Summary | CrossRef Full Textual content | Google Scholar
44. Comment R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, et al. Traits and medical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: affect of tumor origin. Clin Most cancers Res. (2013) 19:4079–91. doi: 10.1158/1078-0432.Ccr-12-3847
PubMed Summary | CrossRef Full Textual content | Google Scholar
45. Lee M, Heo SH, Music IH, Rajayi H, Park HS, Park IA, et al. Presence of tertiary lymphoid constructions determines the extent of tumor-infiltrating lymphocytes in main breast most cancers and metastasis. Fashionable Pathol. (2019) 32:70–80. doi: 10.1038/s41379-018-0113-8
46. Schweiger T, Berghoff AS, Glogner C, Glueck O, Rajky O, Traxler D, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid constructions in pulmonary metastases from colorectal most cancers. Clin Exp Metastasis. (2016) 33:727–39. doi: 10.1007/s10585-016-9813-y
PubMed Summary | CrossRef Full Textual content | Google Scholar
47. Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, et al. Neogenesis of lymphoid constructions and antibody responses happen in human melanoma metastases. Most cancers Res. (2012) 72:3997–4007. doi: 10.1158/0008-5472.Can-12-1377
PubMed Summary | CrossRef Full Textual content | Google Scholar
48. Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid constructions in most cancers – issues for affected person prognosis. Cell Mol Immunol. (2020) 17:570–75. doi: 10.1038/s41423-020-0457-0
PubMed Summary | CrossRef Full Textual content | Google Scholar
49. Ding GY, Ma JQ, Yun JP, Chen X, Ling Y, Zhang S, et al. Distribution and density of tertiary lymphoid constructions predict medical consequence in intrahepatic cholangiocarcinoma. J Hepatol. (2022) 76:608–18. doi: 10.1016/j.jhep.2021.10.030
PubMed Summary | CrossRef Full Textual content | Google Scholar
50. Zhang C, Wang XY, Zuo JL, Wang XF, Feng XW, Zhang B, et al. Localization and density of tertiary lymphoid constructions affiliate with molecular subtype and medical consequence in colorectal most cancers liver metastases. J Immunother Most cancers. (2023) 11:e006425. doi: 10.1136/jitc-2022-006425
PubMed Summary | CrossRef Full Textual content | Google Scholar
51. Rossi A, Belmonte B, Carnevale S, Liotti A, De Rosa V, Jaillon S, et al. Stromal and immune cell dynamics in tumor related tertiary lymphoid constructions and anti-tumor immune responses. Entrance Cell Dev Biol. (2022) 10:933113. doi: 10.3389/fcell.2022.933113
PubMed Summary | CrossRef Full Textual content | Google Scholar
52. Liang H, Zhang Z, Guan Z, Zheng S, Lou J, Liu W, et al. Follicle-like tertiary lymphoid constructions: A possible biomarker for prognosis and immunotherapy response in sufferers with laryngeal squamous cell carcinoma. Entrance Immunol. (2023) 14:1096220. doi: 10.3389/fimmu.2023.1096220
PubMed Summary | CrossRef Full Textual content | Google Scholar
53. Tamiya Y, Nakai T, Suzuki A, Mimaki S, Tsuchihara Okay, Sato Okay, et al. The impression of tertiary lymphoid constructions on clinicopathological, genetic and gene expression traits in lung adenocarcinoma. Lung Most cancers (Amsterdam Netherlands). (2022) 174:125–32. doi: 10.1016/j.lungcan.2022.11.001
PubMed Summary | CrossRef Full Textual content | Google Scholar
54. Nerviani A, Pitzalis C. Function of chemokines in ectopic lymphoid constructions formation in autoimmunity and most cancers. J Leukocyte Biol. (2018) 104:333–41. doi: 10.1002/JLB.3MR0218-062R
PubMed Summary | CrossRef Full Textual content | Google Scholar
55. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing actions of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. (2002) 169:424–33. doi: 10.4049/jimmunol.169.1.424
PubMed Summary | CrossRef Full Textual content | Google Scholar
57. Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid constructions. Immunity. (2022) 55:115–28.e9. doi: 10.1016/j.immuni.2021.12.007
PubMed Summary | CrossRef Full Textual content | Google Scholar
58. Ukita M, Hamanishi J, Yoshitomi H, Yamanoi Okay, Takamatsu S, Ueda A, et al. CXCL13-producing CD4+ T cells accumulate within the early part of tertiary lymphoid constructions in ovarian most cancers. JCI Perception. (2022) 7:e157215. doi: 10.1172/jci.perception.157215
PubMed Summary | CrossRef Full Textual content | Google Scholar
60. Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in continual irritation and tertiary lymphoid organ formation. Annu Rev Immunol. (2015) 33:715–45. doi: 10.1146/annurev-immunol-032713-120252
PubMed Summary | CrossRef Full Textual content | Google Scholar
61. Zhu G, Nemoto S, Mailloux AW, Perez-Villarroel P, Nakagawa R, Falahat R, et al. Induction of tertiary lymphoid constructions with antitumor perform by a lymph node-derived stromal cell line. Entrance Immunol. (2018) 9:1609. doi: 10.3389/fimmu.2018.01609
PubMed Summary | CrossRef Full Textual content | Google Scholar
62. Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid construction formation and native pathology. Proc Natl Acad Sci United States America. (2019) 116:13490–97. doi: 10.1073/pnas.1905301116
63. Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, et al. Immune mechanisms orchestrate tertiary lymphoid constructions in tumors through cancer-associated fibroblasts. Cell Rep. (2021) 36:109422. doi: 10.1016/j.celrep.2021.109422
PubMed Summary | CrossRef Full Textual content | Google Scholar
64. O’Connor RA, Martinez BR, Koppensteiner L, Mathieson L, Akram AR. Most cancers-associated fibroblasts drive CXCL13 manufacturing in activated T cells through TGF-beta. Entrance Immunol. (2023) 14:1221532. doi: 10.3389/fimmu.2023.1221532
PubMed Summary | CrossRef Full Textual content | Google Scholar
65. Hua Y, Vella G, Rambow F, Allen E, Antoranz Martinez A, Duhamel M, et al. Most cancers immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches by a feed-forward loop. Most cancers Cell. (2022) 40:1600–18. doi: 10.1016/j.ccell.2022.11.002
PubMed Summary | CrossRef Full Textual content | Google Scholar
66. Wang X, Li X, Zhao J, Li Y, Shin SR, Ligresti G, et al. Speedy technology of hPSC-derived excessive endothelial venule organoids with in vivo ectopic lymphoid tissue capabilities. Adv Mater. (2024) 36:e2308760. doi: 10.1002/adma.202308760
PubMed Summary | CrossRef Full Textual content | Google Scholar
69. Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based therapy promotes vascular normalization and tertiary lymphoid construction formation within the therapeutic melanoma microenvironment. J Immunother Most cancers. (2021) 9:e001906. doi: 10.1136/jitc-2020-001906
PubMed Summary | CrossRef Full Textual content | Google Scholar
70. Filderman JN, Appleman M, Chelvanambi M, Taylor JL, Storkus WJ. STINGing the tumor microenvironment to advertise therapeutic tertiary lymphoid construction improvement. Entrance Immunol. (2021) 12:690105. doi: 10.3389/fimmu.2021.690105
PubMed Summary | CrossRef Full Textual content | Google Scholar
71. Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares Okay, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Most cancers Immunol Res. (2014) 2:616–31. doi: 10.1158/2326-6066.Cir-14-0027
PubMed Summary | CrossRef Full Textual content | Google Scholar
72. Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination focusing on HPV16 induces T cell responses that localize in mucosal lesions. Sci Trans Med. (2014) 6:221ra13. doi: 10.1126/scitranslmed.3007323
73. Melssen MM, Pollack KE, Meneveau MO, Smolkin ME, Pinczewski J, Koeppel AF, et al. Characterization and comparability of innate and adaptive immune responses at vaccine websites in melanoma vaccine medical trials. Most cancers Immunol Immunother: CII. (2021) 70:2151–64. doi: 10.1007/s00262-020-02844-w
75. Wen Z, Liu H, Qiao D, Chen H, Li L, Yang Z, et al. Nanovaccines fostering tertiary lymphoid construction to assault mimicry nasopharyngeal carcinoma. ACS Nano. (2023) 17:7194–206. doi: 10.1021/acsnano.2c09619
PubMed Summary | CrossRef Full Textual content | Google Scholar
76. Li C, Clauson R, Bugada LF, Ke F, He B, Yu Z, et al. Antigen-clustered nanovaccine achieves long-term tumor remission by selling B/CD 4 T cell crosstalk. ACS Nano. (2024) 18:9584–604. doi: 10.1021/acsnano.3c13038
PubMed Summary | CrossRef Full Textual content | Google Scholar
80. Kobayashi Y, Watanabe T. Gel-trapped lymphorganogenic chemokines set off synthetic tertiary lymphoid organs and mount adaptive immune responses in vivo. Entrance Immunol. (2016) 7:316. doi: 10.3389/fimmu.2016.00316
PubMed Summary | CrossRef Full Textual content | Google Scholar
81. Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA, et al. Basic ideas of hydrogels: synthesis, properties, and their functions. Polymers. (2020) 12:2702. doi: 10.3390/polym12112702
82. Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell improvement and antibody manufacturing. Nat Protoc. (2017) 12:168–82. doi: 10.1038/nprot.2016.157
PubMed Summary | CrossRef Full Textual content | Google Scholar
83. Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, et al. Ex vivo engineered immune organoids for managed germinal heart reactions. Biomaterials. (2015) 63:24–34. doi: 10.1016/j.biomaterials.2015.06.002
PubMed Summary | CrossRef Full Textual content | Google Scholar
84. Jin XK, Liang JL, Zhang SM, Ji P, Huang QX, Qin YT, et al. Engineering metal-based hydrogel-mediated tertiary lymphoid construction formation through activation of the STING pathway for enhanced immunotherapy. Mater Horizons. (2023) 10:4365–79. doi: 10.1039/D3MH00748K
85. Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the professionals and cons. Cell Communication Signaling: CCS. (2022) 20:44. doi: 10.1186/s12964-022-00854-y
86. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic options of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response standards (irPRC). Ann Oncol: Off J Eur Soc Med Oncol. (2018) 29:1853–60. doi: 10.1093/annonc/mdy218
87. Solar X, Liu W, Solar L, Mo H, Feng Y, Wu X, et al. Maturation and abundance of tertiary lymphoid constructions are related to the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung most cancers. J Immunother Most cancers. (2022) 10:e005531. doi: 10.1136/jitc-2022-005531
PubMed Summary | CrossRef Full Textual content | Google Scholar
88. Shang T, Jiang T, Lu T, Wang H, Cui X, Pan Y, et al. Tertiary lymphoid constructions predict the prognosis and immunotherapy response of cholangiocarcinoma. Entrance Immunol. (2023) 14:1166497. doi: 10.3389/fimmu.2023.1166497
PubMed Summary | CrossRef Full Textual content | Google Scholar
89. Hayashi Y, Makino T, Sato E, Ohshima Okay, Nogi Y, Kanemura T, et al. Density and maturity of peritumoral tertiary lymphoid constructions in oesophageal squamous cell carcinoma predicts affected person survival and response to immune checkpoint inhibitors. Br J Most cancers. (2023) 128:2175–85. doi: 10.1038/s41416-023-02235-9
PubMed Summary | CrossRef Full Textual content | Google Scholar
90. Liu Z, Meng X, Tang X, Zou W, He Y. Intratumoral tertiary lymphoid constructions promote affected person survival and immunotherapy response in head neck squamous cell carcinoma. Most cancers Immunol Immunother: CII. (2023) 72:1505–21. doi: 10.1007/s00262-022-03310-5
91. Italiano A, Bessede A, Pulido M, Bompas E, Piperno-Neumann S, Chevreau C, et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid constructions: a part 2 PEMBROSARC trial cohort. Nat Med. (2022) 28:1199–206. doi: 10.1038/s41591-022-01821-3
PubMed Summary | CrossRef Full Textual content | Google Scholar
92. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in sufferers with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. (2020) 26:1845–51. doi: 10.1038/s41591-020-1086-y
PubMed Summary | CrossRef Full Textual content | Google Scholar
93. Villani A, Potestio L, Fabbrocini G, Troncone G, Malapelle U, Scalvenzi M. The therapy of superior melanoma: therapeutic replace. Int J Mol Sci. (2022) 23:6388. doi: 10.3390/ijms23126388
PubMed Summary | CrossRef Full Textual content | Google Scholar
94. Griss J, Bauer W, Wagner C, Simon M, Chen M, Grabmeier-Pfistershammer Okay, et al. B cells maintain irritation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. (2019) 10:4186. doi: 10.1038/s41467-019-12160-2
PubMed Summary | CrossRef Full Textual content | Google Scholar
95. Ding L, Solar L, Bu MT, Zhang Y, Scott LN, Prins RM, et al. Antigen presentation by clonally various CXCR5+ B cells to CD4 and CD8 T cells is related to sturdy response to immune checkpoint inhibitors. Entrance Immunol. (2023) 14:1176994. doi: 10.3389/fimmu.2023.1176994
PubMed Summary | CrossRef Full Textual content | Google Scholar
96. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung most cancers immunotherapy: progress, pitfalls, and guarantees. Mol Most cancers. (2023) 22:40. doi: 10.1186/s12943-023-01740-y
PubMed Summary | CrossRef Full Textual content | Google Scholar
97. Camidge DR, Doebele RC, Kerr KM. Evaluating and contrasting predictive biomarkers for immunotherapy and focused remedy of NSCLC. Nat Rev Clin Oncol. (2019) 16:341–55. doi: 10.1038/s41571-019-0173-9
PubMed Summary | CrossRef Full Textual content | Google Scholar
98. Brunet M, Crombé A, Cousin S, Vanhersecke L, Le Loarer F, Bessede A, et al. Mature tertiary lymphoid construction is a selected biomarker of most cancers immunotherapy and doesn’t predict consequence to chemotherapy in non-small-cell lung most cancers. Ann Oncol: Off J Eur Soc Med Oncol. (2022) 33:1084–85. doi: 10.1016/j.annonc.2022.06.007
99. Yu A, Cao M, Zhang Okay, Yang Y, Ma L, Zhang X, et al. The prognostic worth of the tertiary lymphoid construction in gastrointestinal cancers. Entrance Immunol. (2023) 14:1256355. doi: 10.3389/fimmu.2023.1256355
PubMed Summary | CrossRef Full Textual content | Google Scholar
100. Küçükköse E, Heesters BA, Villaudy J, Verheem A, Cercel M, van Hal S, et al. Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice. J Immunother Most cancers. (2022) 10:e005345. doi: 10.1136/jitc-2022-005345
PubMed Summary | CrossRef Full Textual content | Google Scholar
101. Groeneveld CS, Fontugne J, Cabel L, Bernard-Pierrot I, Radvanyi F, Allory Y, et al. Tertiary lymphoid constructions marker CXCL13 is related to higher survival for sufferers with advanced-stage bladder most cancers handled with immunotherapy. Eur J Most cancers. (2021) 148:181–89. doi: 10.1016/j.ejca.2021.01.036
PubMed Summary | CrossRef Full Textual content | Google Scholar
102. Meylan M, Sautès-Fridman C, Fridman WH. Tertiary lymphoid constructions generate and propagate anti-tumor antibody-producing plasma cells in renal cell most cancers. Med Sci: M/S. (2022) 38:536–38. doi: 10.1051/medsci/2022069
103. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung most cancers. New Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
PubMed Summary | CrossRef Full Textual content | Google Scholar
104. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung most cancers. New Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983
PubMed Summary | CrossRef Full Textual content | Google Scholar
105. Patel SP, Othus M, Chen Y, Wright GP Jr., Yost KJ, Hyngstrom JR, et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in superior melanoma. New Engl J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
PubMed Summary | CrossRef Full Textual content | Google Scholar
106. Wang Q, Solar Okay, Liu R, Music Y, Lv Y, Bi P, et al. Single-cell transcriptome sequencing of B-cell heterogeneity and tertiary lymphoid construction predicts breast most cancers prognosis and neoadjuvant remedy efficacy. Clin Trans Med. (2023) 13:e1346. doi: 10.1002/ctm2.1346
107. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid constructions promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
109. Lee JW, Park BC, Jang NY, Lee S, Cho YK, Sharma P, et al. Inducing ectopic T cell clusters utilizing stromal vascular fraction spheroid-based immunotherapy to boost anti-tumor immunity. Adv Sci. (2022) 9:e2203842. doi: 10.1002/advs.202203842
110. Aoyama S, Nakagawa R, Mulé JJ, Mailloux AW. Inducible tertiary lymphoid constructions: promise and challenges for translating a brand new class of immunotherapy. Entrance Immunol. (2021) 12:675538. doi: 10.3389/fimmu.2021.675538
PubMed Summary | CrossRef Full Textual content | Google Scholar
111. Houel A, Foloppe J, Dieu-Nosjean MC. Harnessing the facility of oncolytic virotherapy and tertiary lymphoid constructions to amplify antitumor immune responses in most cancers sufferers. Semin Immunol. (2023) 69:101796. doi: 10.1016/j.smim.2023.101796
PubMed Summary | CrossRef Full Textual content | Google Scholar
112. Rosenberg MI, Greenstein E, Buchkovich M, Peres A, Santoni-Rugiu E, Yang L, et al. Polyclonal lymphoid growth drives paraneoplastic autoimmunity in neuroblastoma. Cell Rep. (2023) 42:112879. doi: 10.1016/j.celrep.2023.112879
PubMed Summary | CrossRef Full Textual content | Google Scholar