Persistent myeloid leukemia (CML) is characterised by a reciprocal translocation between chromosome 9 and 22 within the hematopoietic stem cell that ends in formation of the Philadelphia chromosome (Ph), encoding the BCR::ABL1 fusion gene [1]. The formation of the corresponding BCR::ABL1 oncoprotein causes the depletion of the N-terminal cap of Abelson murine leukemia viral oncogene homolog 1 (ABL1), which beneath physiological circumstances binds within the myristoyl pocket of the C-terminal lobe of the kinase area and thereby negatively regulates its exercise [2]. Lack of ABL1 autoregulation contributes to the constitutive activation of BCR::ABL1, pushed by homo-oligomerisation of BCR::ABL1 mediated by the coiled-coil area of the breakpoint cluster area (BCR) protein [3], which in flip induces uncontrolled proliferation and survival of leukemia stem cells. Other than the everyday BCR::ABL1 transcripts e13a2 and e14a2, lower than 2% of sufferers categorical atypical transcripts reminiscent of e1a2, e8a2, or e19a2. In these instances, nevertheless, dependable monitoring by routine real-time quantitative polymerase chain response (RT-qPCR) will not be possible, subsequently an evaluation of the person molecular response with particular RT-qPCR primers is advisable [4, 5].
The arrival of tyrosine kinase inhibitors (TKIs), which competitively disrupt enzyme exercise by binding on the adenosine triphosphate (ATP)-binding website of BCR::ABL1, has considerably improved final result of CML sufferers and is now normal of care. For first-line therapy of CML in persistent section (CML-CP), imatinib in addition to second-generation TKIs (2GTKIs) dasatinib, nilotinib, and bosutinib are advisable [6]. In comparison with imatinib, 2GTKIs obtain earlier and deeper molecular response, allowing treatment-free remission (TFR) extra often. Nevertheless, off-target inhibition could also be related to a definite side-effect profile [6]. Within the European Cease Tyrosine Kinase Inhibitor (EURO-SKI) trial, 46% of sufferers confirmed a significant molecular response (MMR) 3 years after discontinuation of therapy. To this point, as a result of want for standardized evaluation of residual illness, solely sufferers with typical transcripts have been investigated in discontinuation research. The e14a2 BCR::ABL1 transcript is favorably related to the success of long-term TFR [7].
Resistance and intolerance to ATP-competitive TKIs have been described in about 25% of instances [8, 9]. T315I mutations happen in round 5% of sufferers handled with 2GTKIs and confer resistance by restoring or growing ABL1 kinase exercise [9, 10]. Ponatinib, a third-generation TKI, and allogeneic stem cell transplantation had been for a few years the one profitable therapy choices [6, 11]. Just lately, with asciminib, a TKI with a brand new mode of motion has develop into obtainable, particularly focusing on the ABL myristoyl pocket (STAMP) impartial of ATP website mutations [12]. Resulting from its superior efficacy and enhanced tolerability, asciminib was lately authorized for the therapy of persistent section CML after failure or intolerance of no less than two TKIs [12], or, in some international locations, for sufferers with T315I mutation [13].
Right here we report on a younger affected person with CML who was, after TKI resistance with T315I mutation and subsequent intolerance to ponatinib, reluctant to try allogeneic stem cell transplantation. The affected person determined as a substitute for participation in a section I trial with asciminib and achieved a sustained therapy response with undetectable e19a2 BCR::ABL1 transcripts, allowing a profitable discontinuation try. The scientific trial has been authorized by the accountable ethics committee. Affected person samples investigated on this research had been obtained after knowledgeable consent and based on the Helsinki Declaration. The affected person supported the evaluation and agreed with the publication.
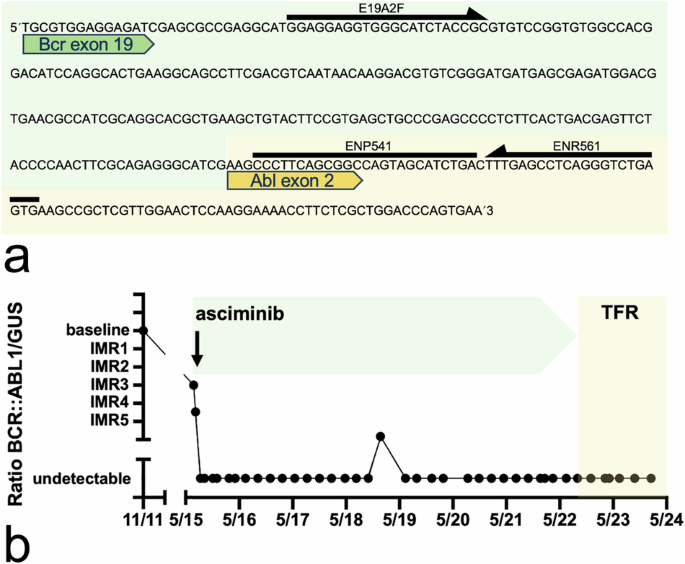
In November 2011, the 33-year-old male affected person was identified with CML with leukocytosis of 99 × 109/L with 21% basophils, 13% blasts, 774 × 109/L platelets, splenomegaly 3 cm under left costal margin and cytogenetic proof of the Philadelphia chromosome. Bone marrow was in keeping with CML in CP with marked eosinophilia (Fig. 1a). Further chromosomal abnormalities weren’t detected. Multiplex-PCR [14] revealed the atypical BCR::ABL1 transcript e19a2 (Fig. 1b). He was at intermediate danger based on the EUTOS long-term survival (ELTS) rating. To specify the molecular follow-up of the atypical transcript e19a2, the BCR::ABL1 fusion was confirmed by Sanger sequencing, and particular qPCR primers had been utilized to watch response (Fig. 2a). BCR::ABL1 transcript ranges had been calculated in relation to beta-glucuronidase (GUSB) transcripts and the ratios obtained over time had been in comparison with the ratio at prognosis with a purpose to decide the person molecular response (IMR) to asciminib [4] (Fig. 2b). Following first-line therapy with nilotinib inside the ENEST1st trial (NCT01061177), a person discount of BCR::ABL1 transcripts [4] by three log, approximating to an MMR, was achieved after 3 months. A four-log discount was achieved after 9 months. Nevertheless, lack of molecular and cytogenetic response occurred after 30 months; upon detection of a T315I mutation, second-line remedy with ponatinib was commenced. Prompted by newly identified arterial hypertension, the dose of ponatinib was lowered from 45 to 30 mg day by day. After 4 months of ponatinib, a three-log discount was achieved once more. Remedy was prolonged by including pegylated interferon alfa-2b 35 µg weekly after 9 months with the goal of enhancing illness management, however with out success.
(a) Bone marrow reveals hypercellularity with maturing myelopoiesis and elevated basophil and eosinophil granulocytes in keeping with CML in persistent section, scale bar = 10 µm. (b) Qualitative multiplex PCR revealed in lane 5 the atypical transcript e19a2 of the affected person (925 bp). BCR bands (808 bp) are inner optimistic controls in lanes 1 to five. L- ladder (HSD1000 tapestation). M—DNA free grasp combine; lane 1—adverse management; lane 2—SD1 cell line (BCR::ABL1 transcript e1a2, 481 bp); lane 3—K562 cell line (BCR::ABL1 transcript e14a2, 385 bp); lane 4—BV173 cell line (BCR::ABL1 transcript e13a2, 310 bp); marker—higher and decrease tapestation marker.
(a) Following Sanger sequencing of the e19a2 transcript, the ahead primer E19A2F (5′-GGA GGA GGT GGG CAT CTA CCG-3′) and the traditional reverse primer ENR561 (5′-CAC TCA GAC CCT GAG GCT CAA-3′) with the ENP541 (5′-CCC TTC AGC GGC CAG TAG CAT CTGA-3′) probe had been used for MRD evaluation by RT-qPCR. (b) To calculate the person molecular response (IMR), log discount throughout therapy with asciminib was decided by comparability to the ratio BCR::ABL1/GUSB [4]. ENP European community TaqMan probe, ENR European community reverse primer, Bcr breakpoint cluster area, Abl Abelson murine leukemia viral oncogene homolog; GUSB – beta-glucuronidase; TFR treatment-free remission.
Because of the affected person’s younger age and cardiovascular unwanted side effects of ponatinib [15], donor search was initiated and an allogeneic stem cell transplantation was mentioned. Presently, the multicenter section 1 trial of oral asciminib (ABL001) for sufferers with CML or Ph+ acute lymphoblastic leukemia (NCT02081378) was recruiting. After knowledgeable consent balancing the dangers of transplantation vs. potential transformation of CML, therapy was switched to third-line asciminib 150 mg twice day by day based on research randomization. Asciminib was properly tolerated and after 4 weeks of therapy no measurable residual illness was detectable with five-log particular person PCR sensitivity. After 5 months of remedy, grade 1 improve in serum lipase and amylase with out scientific or radiologic correlates of pancreatitis occurred for the primary time, in order that after a 2-week break and normalization of enzyme actions, the dose of asciminib was lowered to 80 mg twice day by day. Molecularly undetectable illness with five-log sensitivity continued, cardiovascular signs related to ponatinib had disappeared. Throughout the next therapy interval of seven.3 years with asciminib, a sustained therapy response with nearly persistently undetectable residual illness of CML was revealed in three-month PCR controls. Though there are not any suggestions for CML with atypical transcripts, a discontinuation try was made after a shared decision-making with the affected person. Inside 18 months after therapy discontinuation, RT-qPCR monitoring didn’t reveal any proof of BCR::ABL1 expression with steady five-log PCR sensitivity (GUSB transcript vary 275,037–4,038,847) (Fig. 2b).
Normally, CML sufferers with T315I mutation have an unfavorable prognosis. Nevertheless, long-term final result depends upon section of illness when T315I mutation happens, with 5-year general survival estimated at 70% in CP and 10% in blast section [9, 11]. Despite the fact that ponatinib is extremely efficient in sufferers with T315I mutation, cardiovascular or thromboembolic unwanted side effects typically necessitate dose modifications or discontinuation of remedy. The frequency of arterial hypertension as ponatinib-associated aspect impact, main to alter of therapy within the reported case, is reported to be 37% [15]. Resulting from its particular mechanism of motion, asciminib reveals fewer off-target results and stays efficient at larger doses even with T315I mutation [13]. Nevertheless, obtainable knowledge on asciminib in sufferers with CML and T315I mutation are nonetheless restricted. Within the section I scientific trial investigating asciminib monotherapy after prior therapy with no less than one TKI, nearly half of the sufferers with CML-CP and T315I mutation achieved MMR. Amongst ponatinib-naïve sufferers, about 68% achieved MMR in opposition to 35% of these pretreated with ponatinib. In sufferers switching from ponatinib to asciminib resulting from intolerance, MMR charges had been notably larger than in those that had been resistant, with round 57% and 15%, respectively. Nevertheless, this evaluation excluded three sufferers resulting from an atypical BCR::ABL1 transcript, together with the case reported right here [13]. When switching to asciminib, the ratio BCR::ABL1/GUSB of this affected person was 0.010% and T315I mutation was not detectable by Sanger sequencing. The described asymptomatic improve in lipase and amylase has been reported in 29% and 12% of sufferers handled with asciminib on the larger dose and in 5% and 6% of sufferers handled with the common dose (80 mg day by day), respectively [12, 13]. Following the research protocol, the dose was lowered within the reported case, however with none adverse impression on therapy response. Based on the European LeukemiaNet 2020 suggestions, therapy discontinuation in sufferers with atypical transcripts will not be suggested if quantitative PCR controls are routinely carried out [6]. Nevertheless, discontinuation could be rigorously monitored in extremely specialised laboratories in order that samples from eligible sufferers must be allotted to them [4, 5]. Sequencing of the atypical BCR::ABL1 transcript must be carried out on a pre-treatment pattern.
That is the primary report of a CML affected person with an atypical e19a2 BCR::ABL1 transcript and T315I mutation in whom therapy with asciminib resulted in steady full molecular remission with 5 log PCR sensitivity (MR5). In distinction to present suggestions [5], asciminib was discontinued after 7 years in particular person MR5. Quantitative monitoring of residual illness was possible utilizing a person method and permitted the TFR try even after T315I triggered resistance. With the intention to present entry to revolutionary therapy choices and to gather knowledge on particular person response patterns, sufferers with atypical BCR::ABL1 transcripts who’re doubtlessly eligible for therapy cessation must be thought-about in potential scientific trials.