Sufferers and tissue assortment
We extracted RNA and carried out RNAseq of tissue samples from 298 BCs enrolled in two translational research (Fig. 1). Resulting from high quality management failure in a single pattern, we evaluated RNAseq knowledge from 297 early BCs.
Baseline traits of tissue samples are described in Desk 1. In 297 BCs. median age at analysis of BC sufferers was 39.9 years (interquartile vary [IQR]: 35.5–49.4); 81.1% of samples have been collected in tissue biopsy in neoadjuvant (NAC) setting and 18.9% surgical specimens in an adjuvant setting. All BC specimens have been harvested from the breast. With regard to BC subtype, 51.2% had triple-negative BC (TNBC), 22.6% HR + HER2-, 14.1% HR + HER2 + , and 12.1% HR-HER2 + . In NAC setting, 27.4% have been HR + HER2-BC, 32.4% in HER2+ no matter HR state and 40.2% in TNBC whereas 98.2% of younger breast most cancers(YBC) cohort have been TNBC. We additional evaluated the intrinsic subtype via PAM50 analyses: luminal A kind was recognized in 17.5% of samples, luminal B in 10.1%, basal-like in 49.5%, HER2 enriched in 18.5%, and regular in 4.4%.
Amongst 297 BCs, we collected comply with up survival knowledge and therapy info in 197 BC sufferers (Desk 1). In 197 BC sufferers, 161 sufferers have been handled with NAC adopted by healing surgical procedure and 52 sufferers have been handled with surgical procedure adopted by adjuvant therapy relating to BC subtypes. In 161 BCs in NAC cohort, medical stage at analysis included 19.3% of medical stage IIIC and 57.4% of sufferers have been recognized as TNBC (Desk 1). Particulars of BC subtypes and intrinsic subtypes have been described in Supplementary Tables 2 and 3.
Fusions in line with BC traits
We analyzed the RNAseq of 297 tissue samples utilizing three fusion detection software program packages (Supplementary Desk 4). First, we included fusions with greater than three supporting reads (Fig. 1). We excluded fusions recognized by just one program, artifacts, and fusions present in regular tissue.
We discovered a median of 5 to eight fusions (Supplementary Desk 4). Among the many three callers, STAR.Arriba detected essentially the most fusions (median variety of fusions: 8, IQR: 4–14), whereas STAR.Fusion (median: 7, IQR: 4–13) and STAR.SEQR (median: 5, IQR: 3–9) discovered the fewest. The median variety of detected fusions per BC pattern after filtering was 5 (IQR: 3–9) (Supplementary Desk 5).
We additionally evaluated fusions in line with BC subtype (Fig. 2a). HR + HER2- BC had the fewest fusions (median: 7, IQR: 3–15) in comparison with TNBC (median: 9, IQR: 4.75–14), HR-HER2 + BC (median: 9.5, IQR: 5.75–18.25), and HR + HER2 + BC (median: 10, IQR: 6–13) (p = 0.16). In intrinsic subtype, the normal-like subtype had the fewest fusions (median: 1, IQR: 0, 3) adopted by the luminal A (median: 5.5, IQR: 2.75, 10.25), luminal B (median: 9, IQR: 6, 16.5), HER2-enriched (median: 9, IQR:6, 16.5) and basal-like (median 10, IQR: 6, 15.5) intrinsic subtypes, in ascending order (p < 0.05) (Fig. 2b).
Additional intrinsic subtype evaluation introduced that basal-like subtype had extra fusions in comparison with different intrinsic subtypes in HR + HER2- BC (median variety of fusions of basal like subtype in HR + HER2-BC: 17.5, IQR:9.75–18.75) (Fig. 2c) and TNBC (median quantity:10, IQR: 6 -15) (Fig. 2f) (p < 0.05, respectively). Nonetheless, in HER2 + BC no matter HR standing, there was no distinction in variety of fusions in line with intrinsic subtype (ps > 0.05, respectively) (Fig. 2nd, e).
Different genomic traits together with homologous recombinant deficiency (HRD) rating, tumor mutational burden(TNB) rating, and replica quantity variant (CNV) have been additionally evaluated for affiliation with variety of fusions utilizing 126 BCs which being carried out entire exome sequencing (WES) evaluation (Fig. 3a–c and supplementary Desk 2 and 3). On this evaluation, excessive HRD rating, excessive TMB rating and excessive CNV burden have been positively correlated to variety of fusions (p = 0.010, p = 0.003, and p = 0.035, respectively). In subspecific analyses in line with BC subtype, CNV burden was related to variety of fusions in HR + HER2- subtype and TNBC whereas HRD was in HR-HER2+ subtype (Fig. 3d–o).
a Homologous recombinant deficiency (HRD) rating, b Tumoral mutational burden (TMB). c Copy quantity variant (CNV burden) between excessive (n = 45) and low (n = 81) variety of fusions in all breast most cancers (BC) (n = 126 which being carried out each WTS and entire exome sequencing), d HRD rating, e TMB. f CNV burden between excessive (n = 10) and low (n = 23) variety of fusions in HR + HER2- BC, g HRD rating. h TMB. i CNV burden between excessive (n = 7) and low (n = 16) variety of fusions in HR + HER2 + BC. j HRD rating. ok TMB, l CNV burden between excessive (n = 8) and low (n = 8) variety of fusions in HR-HER2 + BC. m HRD rating. n TMB. o CNV burden between excessive (n = 20) and low (n = 34) variety of fusions in TNBC.
Frequent fusions in early breast most cancers
After filtering, we discovered 2439 distinctive fusions (Desk 2). Amongst these occasions, there have been 515 (21.1%) recurrent occasions, 365 (15.0%) recognized cancer-related fusions and 131 (5.4%) recognized BC-related fusions in line with public databases together with Mitelman and FusionAnnotator, which contained ChimerDB, COSMIC, and TCGA fusions22,23. With regard to chromosomes, chromosome 17 had most fusions adopted by chromosome 1, 11, and eight amongst 23 chromosomes. As well as, intrachromosomal fusion was detected extra continuously than interchromosomal fusion (Desk 3). The 4 chromosomes harboring essentially the most fusions additionally harbored as much as 70% of intrachromosomal fusions. FBXL20, BCAS3, ERBB2, and IKZF3 have been essentially the most continuously detected fusion genes in chromosome 17. In whole, 77 (3.2%) fusions have been recognized recurrent fusions. Probably the most generally detected fusion occasion was FSIP1-AC013652.1 (Supplementary Desk 6).
We additionally analyzed fusions in chromosomes in line with BC subtype and intrinsic subtype (Fig. 4). In BC subtype, the fusions in HR + HER2 + BC subtype largely occurred in chromosome 17, whereas the fusions in TNBC have been largely noticed in chromosome 1 however evenly distributed in entire chromosomes (p < 0.05) (Fig. 4a, c). In intrinsic subtypes, fusions within the basal-like subtypes largely occurred in chromosome 1, whereas the opposite occurred in chromosome 17, respectively (p < 0.05) (Fig. 4b, d).
a The proportion of fusions in chromosomes in line with HR + HER2- breast most cancers (BC), HR + HER + BC, HR-HER2 + BC and triple destructive breast most cancers (TNBC), b The proportion of fusions in chromosomes in line with luminal A, luminal B, HER2-enriched, basal and regular intrinsic subtype, c Circos plot for fusions in line with HR + HER2- BC, HR + HER + BC, HR-HER2 + BC and TNBC, d Circos plot for fusions in line with luminal A, luminal B, HER2-enriched, basal and regular intrinsic subtype.
Survival outcomes in line with variety of fusions
We additional evaluated therapy outcomes in line with fusions (Fig. 5). Solely 197 BC sufferers having comply with up survival knowledge have been enrolled on this evaluation. For, survival evaluation, we divided BCs into two teams in line with variety of fusions with a 0.6 cut-off worth (11 fusions). The 0.6 lower off worth was based mostly on log-rank check with consecutive cutoff values in whole BC sufferers and this was numerically 11 fusions (Supplementary Desk 7).
a Variety of fusions between non-pathologic full response(pCR) (n = 109) and pCR (n = 52) in all subtypes, b Variety of fusions between non-pathologic full response(pCR) (n = 31) and pCR (n = 7) in HR + HER2- BC. c Variety of fusions between non-pathologic full response(pCR) (n = 13) and pCR (n = 13) in HR + HER2 + BC. d Variety of fusions between non-pathologic full response(pCR) (n = 10) and pCR (n = 10) in HR-HER2 + BC and (e) variety of fusions between non-pathologic full response(pCR) (n = 55) and pCR (n = 22) in triple-negative breast most cancers (TNBC). f Kaplan-Meier (KM) for event-free survival (EFS) in line with excessive vs. low variety of fusions (cut-off worth: 0.6) in all subtypes (n = 197). g KM for EFS in HR + HER2- BC (n = 38), h KM for EFS in HR + HER2 + BC (n = 26). i KM for EFS in HR-HER2 + BC (n = 20) and (j) KM for EFS in TNBC (n = 113).
Amongst these 197 sufferers, 143 sufferers in NAC cohort have been evaluated for pathologic full response(pCR) in line with fusions (Fig. 5a–e). On this evaluation, the variety of fusions was not considerably totally different in line with the pCR standing with all sufferers (p = 0.23, Fig. 5a). In BC subtype, larger variety of fusions was noticed in pCR group in comparison with non-pCR group (p = 0.031) in HR-HER2 + BC subgroup (Fig. 5d) whereas HER2-enriched intrinsic subtype with pCR had decrease fusions in comparison with these with non-pCR (p = 0.021) (Supplementary Fig. 1C). Furthermore, luminal A subtype had larger fusions in these with pCR in comparison with these with out pCR (p = 0.045) (Supplementary Fig. 1B). In any other case, there was no vital distinction of the variety of fusions by pCR standing in different BC subtypes and intrinsic subtypes (Fig. 5b–e and supplementary Fig. 1B, D)
Moreover, we carried out survival evaluation in 197 EBC sufferers with 7 years of median comply with up period (Fig. 5f–j). The five-year event-free survival (5Y-EFS) charge was 75.6% in all sufferers (95% confidence interval [CI]: 0.699, 0.819) and the 5Y-EFS charge was 68.1% within the excessive fusion group (n = 72) and 80.0% within the low fusion group (n = 125) (p = 0.024) (Fig. 5f).
In survival evaluation for fusions in line with BC subtypes, TNBC with larger variety of fusions (n = 43) had a 5Y-EFS of 65.1%, and that with low fusions, 85.7% (n = 70) (p = 0.013), whereas their 5Y-EFS was 77.9% (95% CI: 0.706, 0.859) (n = 113) (Fig. 5j). In non-TNBCs, they’d a pattern that prime fusions have been related to poor EFS, however statistical significance was not noticed (Fig. 5g–i).
Amongst 5 intrinsic subtypes, we analyzed EFS in line with the fusions in 4 intrinsic subtypes as a result of normal-like intrinsic subtype was solely 5. Within the basal-like intrinsic subtype (n = 112), the five-year EFS charge was 78.6% (95% CI: 0.713, 0.866). The basal–excessive fusion group had five-year EFS of 64.6% (n = 40) versus 89.1% within the basal–low fusion group (n = 72) (p = 0.003) (Supplementary Fig. 1H) in the meantime there have been no relationship between fusions and EFS amongst non-basal like intrinsic subtypes (Supplementary Fig. 1E–G)
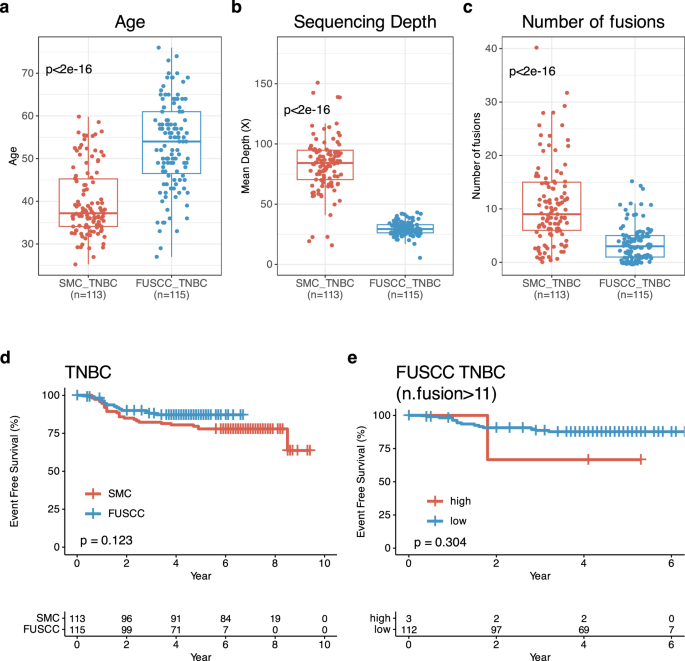
For validating our knowledge, we evaluated the affiliation between fusions and 5Y-EFS charge in TNBC and basal-intrinsic subtypes utilizing TNBC RNASeq knowledge from Fudan College Sanghai Most cancers Middle (FUSCC). In whole, we might use 115 TNBC RNASeq knowledge from FUSCC TNBC cohort. On this validation cohort, median age of sufferers at BC analysis was 54.0 (IQR: 46.5, 61.0) and solely twelve sufferers have been underneath 40 years of age (p < 0.005) (Fig. 6a). When it comes to imply depth of sequencing, FUSCC cohort had decrease than our cohort (p < 0.005) and fewer fusions in comparison with our TNBC (median fusions: 3, IQR: 1, 5) (p < 0.005) (Fig. 6b, c). When it comes to different medical traits, we can’t discover therapy setting relating to neoadjuvant and adjuvant settings.
Validation research with Fudan College Sanghai Most cancers Middle (FUSCC) TNBC. Comparability of (a). age between SMC TNBC at analysis (n = 113)_ and FUSCC TNBC at surgical procedure (n = 115). b Entire transcriptome sequencing depth in SMC TNBC (n = 113) and FUSCC TNBC (n = 113). c Variety of fusions depth in SMC TNBC (n = 113) and FUSCC TNBC (n = 113). d Kaplan-Meier(KM) of event-free survival(EFS) in line with SMC (n = 113) and FUSCC TNBCs (n = 115). e KM for EFS of FUSCC TNBC in line with excessive vs. low variety of fusions (SMC cutoff worth:0.6, n = 115).
There have been related EFS between FUSCC and our cohorts (Fig. 6d). Solely three TNBCs had as much as eleven fusions and due to this fact no vital EFS distinction was noticed (p = 0.304) regardless that three had decrease 5Y-EFS charge in comparison with others in FUSCC cohort (Fig. 6e). Additional analyses utilizing totally different lower off values of fusions have been carried out in FUSCC cohort and the outcomes confirmed persistently that extra fusions in TNBCs was the surrogate marker of shorter EFS in comparison with these with fewer fusions (Supplementary Fig. 3).
Immune standing in line with fusions
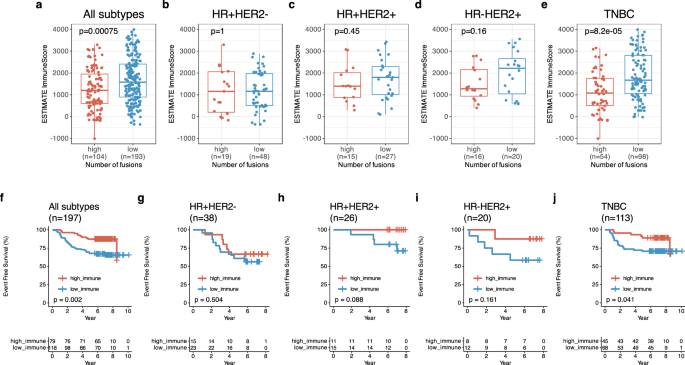
Afterwards, we carried out the evaluation for the affiliation between ESTIMATE ImmuneScore and the variety of fusions. On this evaluation, excessive fusion group had a decrease ImmuneScore than low fusion group with all sufferers (p < 0.001) (Fig. 7a). The TNBC–excessive fusion group had a decrease ImmuneScore (median: 1079, IQR: 514, 1761) than the TNBC–low fusion group (median: 1673, IQR: 1057, 2809) (p < 0.001) (Fig. 7e) however non-TNBC subtype didn’t have a relationship (Fig. 7b–d). Likewise, basal-like intrinsic subtype had a relationship between ImmuneScore and variety of fusions (p = 0.0016, Supplementary Fig. 2D) however there was no relationship in non-basal intrinsic subtypes (Supplementary Fig. 2A–C). In survival evaluation for ImmuneScore, excessive ImmuneScore group had higher EFS than low ImmuneScore group (p = 0.002, Fig. 7f). TNBC sufferers with a excessive ImmuneScore had higher EFS in comparison with that with a low ImmuneScore (five-year EFS of TNBC with excessive vs. low ImmuneScore: 91.9% [95% CI: 0.835, 1.00] vs. 71.1% [95% CI: 0.616, 0.820]) (Fig. 7j). This pattern was additionally noticed in basal-like intrinsic subtypes (p = 0.019, Supplementary Fig. 2H). Withal, ImmuneScore didn’t affected EFS in non-TNBCs (Fig. 7g–i) in addition to non-basal intrinsic subtypes (Supplementary Fig. 2E–G).
a ESTIMATE ImmuneScore between excessive (n = 104) and low (n = 193) fusion occasions in all subtypes. b ESTIMATE ImmuneScore between excessive (n = 19) and low (n = 48) fusion occasions in HR + HER2- BC, c ESTIMATE ImmuneScore between excessive (n = 15) and low (n = 27) fusion occasions in HR + HER2 + BC. d ESTIMATE ImmuneScore between excessive (n = 16) and low (n = 20) fusion occasions in HR-HER2 + BC and (e) ESTIMATE ImmuneScore between excessive (n = 54) and low (n = 98) fusion occasions in triple-negative breast most cancers(TNBC), f Kaplan-Meier (KM) for event-free survival (EFS) in line with ESTIMATE ImmuneScore in all subtypes. g KM for EFS in HR + HER2- BC, h KM for EFS in HR + HER2 + BC. i KM for EFS in HR-HER2 + BC and (j) KM for EFS in TNBC.