NCF4 is an ASC-binding protein and related to colorectal most cancers improvement
To discover the spatiotemporal regulation of ASC speck formation and inflammasome activation, we contaminated main WT and Asc–/– bone marrow-derived macrophages (BMDMs) with the bacterium Francisella novicida to induce AIM2 inflammasome activation after which carried out ASC IP-MS to determine proteins that interacted with ASC (Fig. 1a). We in contrast the IP merchandise between WT BMDMs and Asc–/– BMDMs, and located that the parts of the NADPH oxidase complicated NCF4, NCF1, and NCF2 interacted with ASC. Moreover, NCF4 confirmed a a lot larger specificity for ASC than both NCF1 or NCF2 (Fig. 1b and Supplementary Knowledge 1). An evaluation of NCF4, NCF1, and NCF2 homology revealed that the PX area was shared amongst all three proteins, and the PB1 area was carried by NCF4 and NCF2 (Supplementary Fig. 1a). The interplay between ASC and NCF4 was confirmed by co-IP evaluation in HEK293T cells transfected with ASC and Flag-tagged full-length NCF4 and NCF4 fragments (Fig. 1c). Notably, the PX and PB1 domains, however not the SH3 area, have been required for NCF4 and ASC interplay (Fig. 1c). The PYD area however not the CARD area of ASC interacted with NCF4 (Fig. 1d). ASC-NCF1 and ASC-NCF2 interactions have been additionally confirmed in HEK293T cells by co-IP evaluation. NCF1 interacted with the PYD area of ASC, and NCF2 interacted with each the PYD and CARD domains of ASC (Supplementary Fig. 1b, c).
a Immunoblot evaluation of ASC from the immunoprecipitated merchandise generated with immunoprecipitation with an ASC antibody from the lysates of WT and Asc–/– BMDMs contaminated with F. novicida (100 MOI) for 12 h. b Mass spectrometry evaluation of the IP product in (a). The peptides of ASC, NCF4, NCF1, and NCF2 have been detected within the IP product with ASC antibody from WT and Asc–/– BMDMs in two repeated experiments. c Immunoblot evaluation of Myc-ASC co-IP with FLAG-NCF4, FLAG-NCF4-PX, FLAG-NCF4-SH3 and FLAG-NCF4-PB1 from lysates of HEK293T cells transfected with the indicated plasmids. d Immunoblot evaluation of FLAG-NCF4 co-IP with V5-ASC, V5-ASC-PYD, and V5-ASC-CARD from lysates of HEK293T cells transfected with the indicated plasmids. e Co-IP evaluation of endogenous ASC interacting with complete and phosphorylated NCF4, NCF1, and NCF2 in WT and Asc–/– BMDMs handled with the NLRP3 activator LPS plus ATP (LPS, 500 ng/mL for 4 h and ATP, 5 mM for 15 min). f Gene expression evaluation of NCF1, NCF2, and NCF4 in complete (left) and paired (proper, n = 41) colorectal tumors (n = 480) and management tissues (n = 41) from 480 colorectal most cancers (CRC) sufferers of pooled colon and rectal adenocarcinoma datasets within the TCGA database. g Correlation evaluation between the gene expression of NCF4 and survival fee of CRC sufferers. (Excessive, n = 466; Low, n = 131) Knowledge are from 2 (a, b) or consultant of three unbiased experiments with related outcomes (c–e). Wilcoxon signed rank take a look at for (f), Log-rank (Mantel–Cox) take a look at for (g), p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
We subsequent examined the expression and activation of those three proteins in BMDMs with or with out stimulation. Apparently, the phosphorylation of NCF4 and NCF1, however not that of NCF2, was markedly elevated within the presence of LPS, ATP or a mixture of LPS and ATP (Supplementary Fig. 1d). To find out the endogenous interplay between ASC and NCF proteins, we handled WT and Asc–/– BMDMs with NLRP3-activating stimuli (LPS and ATP) with quick time to keep away from formation of insoluble ASC aggregation30, and carried out anti-ASC IP for western blot evaluation. Our outcomes revealed that the ASC-interacting complicated included NCF1, NCF2, and NCF4 in NLRP3-activated WT BMDMs (Fig. 1e).
NCF4 is a susceptibility gene that’s considerably related to Crohn’s illness and colorectal most cancers, though the small print of the mechanism stay unknown31,32. We carried out bioinformatic evaluation to evaluate the gene expression of NCF4, NCF1, and NCF2 in colon tumors and management tissues from 480 colorectal most cancers (CRC) sufferers and 41 paired tumors and related regular tissues obtainable within the TCGA database. Apparently, the expression of NCF4 and NCF1, however not that of NCF2, was considerably downregulated in tumor tissues in comparison with that in management tissues (Fig. 1f). Moreover, an extended five-year survival fee for colorectal most cancers sufferers correlated with a better expression of NCF4, however not NCF1 or NCF2 (Fig. 1g and Supplementary Fig. 1e). Thus, these information point out that the NADPH oxidase parts NCF4, NCF1, and NCF2 type a fancy with ASC throughout inflammasome activation, and these three proteins might play distinct roles in ROS manufacturing and inflammasome activation.
NCF4 contributes to each NLRP3 and AIM2 inflammasome activation
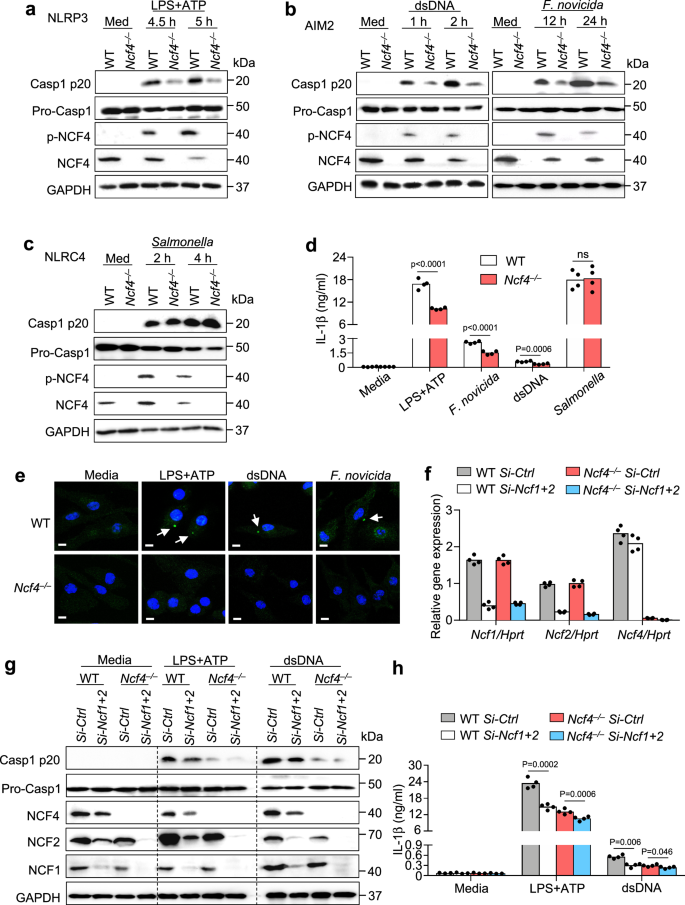
To judge the requirement of NCF1, NCF2, and NCF4 in inflammasome activation, we transfected WT BMDMs with gene-specific siRNAs to knockdown Ncf1, Ncf2, and Ncf4 gene expression after which handled these BMDMs with completely different inflammasome activators (Supplementary Fig. 2a). Notably, the degrees of activated caspase-1 and launched IL-1β in response to LPS and ATP, which activate the NLRP3 inflammasome; dsDNA transfection and an infection with F. novicida, each as activators of the AIM2 inflammasome, have been diminished following transfection with Ncf1, Ncf2, or Ncf4 siRNAs. This discount was most distinguished in Ncf4 siRNA-transfected BMDMs (Supplementary Fig. 2b, c, e). In distinction, caspase-1 activation and IL-1β launch in response to Salmonella enterica Typhimurium (Salmonella) an infection, which prompts the NLRC4 inflammasome, weren’t inhibited in cells handled with siRNA focusing on Ncf1, Ncf2, or Ncf4 genes (Supplementary Fig. second, e).
To offer genetic proof for the position of NCF4 in inflammasome activation, we generated Ncf4–/– mice (Supplementary Fig. 2f, g), which have been viable with traits much like these in WT mice below regular situations, according to a earlier examine33. Importantly, caspase-1 activation and IL-1β launch in response to NLRP3 and AIM2 activation, however not NLRC4 activation, have been considerably diminished in Ncf4–/– BMDMs in comparison with WT BMDMs (Fig. 2a–d and Supplementary Fig. 2h). Moreover, ASC speck formation in response to NLRP3 and AIM2 activators was inhibited within the absence of NCF4 (Fig. 2e). To analyze the potential redundant roles of NCF1, NCF2, and NCF4 in inflammasome activation, we transfected WT and Ncf4–/– BMDMs with siRNAs towards the Ncf1 and Ncf2 genes (Fig. 2f). Caspase-1 activation and IL-1β launch in response to NLRP3 and AIM2 activation have been additional attenuated in Ncf4–/– BMDMs transfected with siRNAs towards each Ncf1 and Ncf2 (Fig. 2g, h). Total, these outcomes point out that NCF4, with contributions from NCF1 and NCF2, performs crucial roles in driving the activation of each NLRP3 and AIM2 inflammasomes.
a–c Immunoblot evaluation of pro-caspase-1 (Professional-Casp1), its subunit p20, complete and phosphorylated NCF4 in WT and Ncf4–/– BMDMs with out remedy (Med) or stimulated with LPS (500 ng/mL, 4 h) and ATP (5 mM, 30 min and 60 min) for NLRP3 inflammasome activation (a), transfected with dsDNA (1.5 μg, 1 h and a couple of h) or contaminated with F. novicida (200 MOI, 12 h and 24 h) for AIM2 inflammasome activation (b), and Salmonella enterica Typhimurium (3 MOI, 2 h and 4 h) for NLRC4 inflammasome activation (c). d Evaluation of IL-1β launch in WT and Ncf4–/– BMDMs with out remedy (Media) or stimulated with LPS (500 ng/mL, 4 h) and ATP (5 mM, 60 min), transfected with dsDNA (1.5 μg, 2 h), contaminated with F. novicida (200 MOI, 24 h), and Salmonella enterica Typhimurium (3 MOI, 4 h) (n = 4 biologically unbiased samples). e Confocal microscopy evaluation of ASC speck formation in WT and Ncf4–/– BMDMs with out remedy or stimulated with LPS (500 ng/mL, 4 h) and ATP (5 mM, 30 min), or transfected with dsDNA (1.5 μg, 1 h); or contaminated with F. novicida (200 MOI, 12 h) as indicated. Arrows point out ASC specks. Scale bars: 10 μm. f–h WT and Ncf4–/– BMDMs have been transfected with siRNAs of Ncf1 and Ncf2, and additional handled with LPS (500 ng/mL, 4 h) and ATP (5 mM, 60 min), and transfected with dsDNA (1.5 μg, 2 h) for inflammasome activation evaluation. f qRT-PCR evaluation of Ncf1, Ncf2, and Ncf4 in WT and Ncf4–/– BMDMs transfected with management siRNA or siRNAs of Ncf1 and Ncf2 (n = 4 technical replicates; 3 unbiased experiments). g, h Immunoblot evaluation of pro-caspase-1 (Professional-Casp1), its subunit p20, NCF4, NCF2, and NCF1 (g), and evaluation of IL-1β launch (h) in WT and Ncf4–/– BMDMs transfected with siRNAs and additional handled with inflammasome stimuli as indicated (n = 4 biologically unbiased samples). Knowledge are from 3 (d, h) or consultant of three unbiased experiments with related outcomes (a–c, e–g). Knowledge symbolize Imply ± SEM for (d, f, h), 2-sided Pupil’s t-test with out multiple-comparisons correction, p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
NCF4 attenuates colorectal most cancers development by way of inflammasome activation
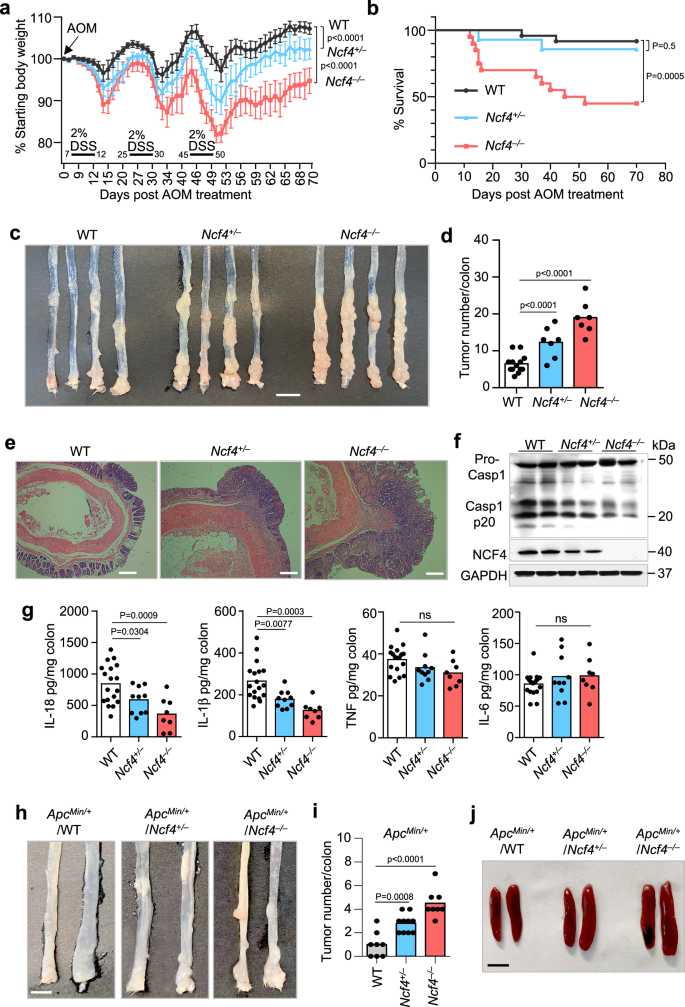
To analyze the position of NCF4 in colorectal most cancers, we intraperitoneally injected littermate WT, Ncf4+/–, and Ncf4–/– mice with AOM on Day 0 adopted by three rounds of DSS remedy (2%) within the consuming water, with evaluation of the tumors within the colons on Day 70 (Fig. 3a). Ncf4–/– mice exhibited essentially the most extreme physique weight reduction after the primary spherical of DSS remedy in comparison with WT and Ncf4+/– mice (Fig. 3a). Solely 45.0% of the Ncf4–/– mice survived past Day 60, whereas 85.7% of the Ncf4+/– mice and 91.7% of WT mice survived (Fig. 3b). We discovered that Ncf4–/– mice developed a considerably greater variety of and bigger tumors than WT and Ncf4+/– mice (Fig. 3c, d and Supplementary Fig. 3a). As well as, the dimensions and weight of the spleen in Ncf4–/– mice have been a lot bigger than these in WT and Ncf4+/– mice (Supplementary Fig. 3b). We noticed extra frequent high-grade dysplasia, elevated severity of the harm, and malignant tumors in Ncf4–/– mice than in WT mice (Fig. 3e). As well as, a morbidity and mortality evaluation revealed that NCF4 exhibited a gene-dose-dependent response to AOM-DSS remedy, and this impact was attribute of haploinsufficient expression (Fig. 3a–e). Likewise, Ncf4–/– mice exhibited extra physique weight reduction and discount of colon size in contrast with WT mice within the DSS-induced colitis mannequin, whereas ROS inhibitors NAC or DPI and APO considerably inhibited the illness development (Supplementary Fig. 3c, d). ROS inhibitors NAC and DPI have been additionally demonstrated to have a constructive impact in attenuating DSS-induced colitis and colitis-associated colorectal most cancers in each mice and people34,35, indicating that NCF4 may stop colorectal most cancers development by means of capabilities apart from ROS alone.
a Physique weight change evaluation of gender- and age-matched WT (n = 14), Ncf4+/– (n = 9), and Ncf4–/– (n = 13) mice after AOM injection at Day 0 and three rounds remedy of DSS (2%). b Survival evaluation of WT (n = 24), Ncf4+/– (n = 14), and Ncf4–/– (n = 20) mice after AOM injection at Day 0 and three rounds of remedy of DSS (2%). c, d Colorectal tumors in WT (n = 13), Ncf4+/– (n = 7), and Ncf4–/– (n = 7) mice day 70 below AOM-DSS remedy. Scale bar: 10 mm. e H&E staining of Colorectal tumors in WT, Ncf4+/–, and Ncf4–/– mice in (c). Scale bars: 10 μm. f, g Immunoblot evaluation of caspase-1 (f) and ELISA evaluation of IL-18, IL-1β, TNF and IL-6 (g) in colon tissues from WT (n = 18), Ncf4+/– (n = 10), and Ncf4–/– mice (n = 8) in (c). h–j Colorectal tumors (h, i) and spleens (j) from gender- and age-matched offspring of ApcMin/+ mice crossed with Ncf4–/– mice with genotype as indicated (n = 8 for WT, n = 10 for Ncf4+/–, and n = 8 for Ncf4–/– in i). Scale bars: 10 mm for (h), and 5 mm for (j). Knowledge are from 2 (b, g, i) or consultant of three unbiased experiments with related outcomes (a, c, d–f, h, j). Knowledge symbolize Imply ± SEM for (d, g, i), 2-sided Pupil’s t-test with out multiple-comparisons correction, two-way ANOVA for (a), Log-rank (Mantel–Cox) take a look at for (b), p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
Importantly, we discovered that caspase-1 activation, IL-18 manufacturing, and IL-1β manufacturing have been considerably decrease in Ncf4–/– mice than in WT and Ncf4+/– mice, whereas the manufacturing of inflammasome-independent cytokines IL-6 and TNF was related between WT, Ncf4+/–, and Ncf4–/– mice (Fig. 3f, g). IL-18 performs a crucial position in mediating safety towards colorectal most cancers pathogenesis36,37. These information point out that impaired inflammasome activation within the Ncf4–/– mice contributes to the larger tumorigenesis noticed. We additional in contrast variations in inflammasome activation and tumor improvement in co-housed WT, Ncf4–/–, and Asc–/– mice. To stop the excessive mortality noticed within the Ncf4–/– mice, we handled the mice with a decrease focus of DSS (1.5%) (Supplementary Fig. 3e). Below this situation, each Ncf4–/– and Asc–/– mice misplaced extra physique weight than the WT mice (Supplementary Fig. 3e). WT mice all survived past Day 70, whereas the survival fee for Ncf4–/– mice was 55.6% and Asc–/– mice was 71.4% (Supplementary Fig. 3f). Each Ncf4–/– and Asc–/– mice introduced with bigger spleens and extra in depth colorectal tumorigenesis than WT mice after AOM-DSS remedy (Supplementary Fig. 3g–i). Moreover, the manufacturing of IL-18 and IL-1β, however not that of IL-6 or TNF, was diminished in Ncf4–/– and Asc–/– mice in comparison with the WT counterparts (Supplementary Fig. 3j). Moreover, in a second unbiased most cancers mannequin, we crossed Ncf4–/– mice with ApcMin/+ mice which spontaneously develop colorectal most cancers, and located that colon most cancers improvement and spleen dimension have been considerably elevated within the absence of NCF4 (Fig. 3h–j). Taken collectively, these information reveal a pivotal position for NCF4 within the prevention of CRC improvement by mediating inflammasome activation.
NCF4 guides the intracellular localization of ASC specks
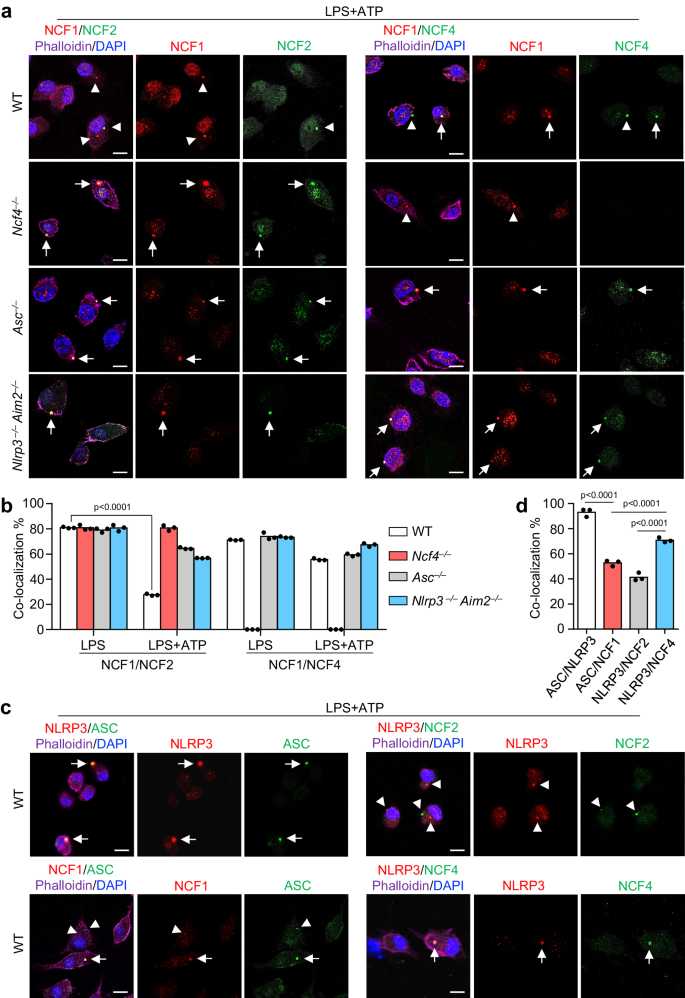
To outline the mechanism by which NCF proteins activate inflammasomes, we analyzed the intracellular localization of NCF1, NCF2, and NCF4 below regular and inflammasome-activating situations. NCF1, NCF2, and NCF4 have been subtle all through untreated WT BMDMs (Supplementary Fig. 4a). Following LPS stimulation in WT, Asc–/–, and Nlrp3–/–Aim2–/– BMDMs, all three proteins exhibited plasma membrane-bound puncta distribution (Supplementary Fig. 4b). The share of punctate-forming cells was roughly 20%, and the colocalization between NCF1 and NCF2 and between NCF1 and NCF4 was roughly 80% (Fig. 4b and Supplementary Fig. 4c). These information point out that NCF1, NCF2, and NCF4 may type equivalent intracellular puncta after LPS stimulation, which is according to the notion that NCF1, NCF2, and NCF4 type a membrane-bound NADPH oxidase after stimulation38. Apparently, sequential LPS and ATP remedy, which triggers NLRP3 inflammasome activation and ASC speck formation, precipitated a outstanding discount in puncta colocalization between NCF1 and NCF2 in WT BMDMs in contrast with the impact of LPS remedy alone (Fig. 4a, b), suggesting that NCF1 and NCF2 are separated in WT BMDMs in response to inflammasome activation. Nevertheless, the colocalization between NCF1 and NCF2 in Ncf4–/–, Asc–/–, and Nlrp3–/–Aim2–/– BMDMs induced by LPS and ATP remedy have been comparable or barely diminished to that induced by LPS alone (Fig. 4a, b), suggesting that the preliminary inflammasome meeting may need a constructive suggestions signaling to drive NCF1 separation from NCF2. The colocalization between NCF1 and NCF4 in WT, Asc–/– and Nlrp3–/–Aim2–/– BMDMs handled with LPS and ATP was solely barely diminished in comparison with that of LPS remedy alone (Fig. 4a, b), suggesting that NCF1 and NCF4 largely keep their spatial localization with each other.
a Confocal microscopy evaluation of the colocalization of NCF1 and NCF2, NCF1, and NCF4 in WT, Ncf4–/–, Asc–/–, and Nlrp3–/–Aim2–/– BMDMs stimulated with LPS (500 ng/mL, 4 h) and ATP (5 mM, 30 min) for NLRP3 inflammasome activation. Arrows point out colocalized puncta; Arrowheads point out separated puncta. Scale bars: 10 μm. b Quantification evaluation of the colocalization of NCF1 and NCF2, NCF1 and NCF4 in WT, Ncf4–/–, Asc–/–, and Nlrp3–/–Aim2–/– BMDMs handled with LPS alone, or LPS plus ATP for inflammasome activation. A minimum of 400 cells for NCF1/NCF2 colocalization evaluation in LPS plus ATP handled teams and 130 (130–200) cells have been analyzed for different teams (n = 3 biologically unbiased samples). c Confocal microscopy evaluation of the colocalization of NLRP3 and ASC, NCF1 and ASC, NLRP3 and NCF2, NLRP3, and NCF4 in WT BMDMs handled with LPS plus ATP for inflammasome activation. Arrows point out colocalized puncta; Arrowheads point out separated puncta. Scale bars: 10 μm. d Quantification evaluation of the colocalization of NLRP3 and ASC, NCF1 and ASC, NLRP3 and NCF2, NLRP3 and NCF4 in (c). A minimum of 130 (130–200) cells have been analyzed for every group (n = 3 biologically unbiased samples). Knowledge are from 3 (b, d) or consultant of three unbiased experiments with related outcomes (a, c). Knowledge symbolize Imply ± SEM for (b, d), 2-sided Pupil’s t-test with out multiple-comparisons correction, p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
The colocalization of ASC specks and puncta shaped by NCF1, NCF2, and NCF4 was analyzed in WT BMDMs. ASC speck formation induced by LPS and ATP remedy was considerably diminished in Ncf4–/–, Asc–/–, and Nlrp3–/–Aim2–/– BMDMs (Supplementary Fig. 4d). Notably, the colocalization between ASC specks and NCF4 puncta was a lot greater than that between ASC specks and NCF1 and NCF2 (Fig. 4c, d). Collectively, these information point out that the punctate formation by NCF4, NCF1, and NCF2 triggered by LPS stimulation may contribute to the intracellular localization of ASC specks induced by sequential LPS and ATP stimulation, and NCF4 performs dominant and important roles within the steering of ASC speck formation.
NCF4 balances NADPH oxidase exercise and inflammasome activation
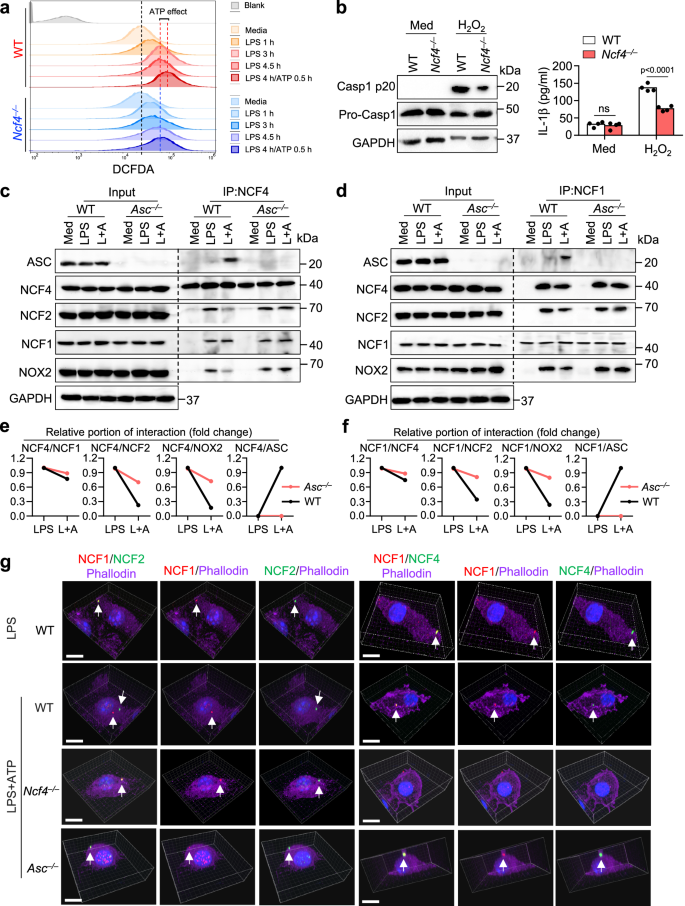
NCF4 is a part of the NADPH oxidase complicated and capabilities within the manufacturing of ROS inside phagocytic cells33. We carried out a DCFDA evaluation and located that LPS plus ATP remedy induced extra ROS manufacturing than LPS alone in WT BMDMs, and that Ncf4–/– BMDMs had a lower in ROS manufacturing in response to LPS plus ATP remedy (Fig. 5a). Moreover, ROS inhibition utilizing inhibitors constantly diminished NLRP3 inflammasome activation triggered by LPS and ATP remedy (Supplementary Fig. 5a, b). To find out whether or not the diminished inflammasome activation in Ncf4–/– BMDMs was as a result of impaired ROS manufacturing, we handled WT and Ncf4–/– BMDMs with H2O2 which rescues the intracellular lower in ROS. Nevertheless, a discount in caspase-1 activation, IL-1β manufacturing, and ASC speck formation of Ncf4–/– BMDMs remained (Fig. 5b and Supplementary Fig. 5c) and that NOX2 inhibition didn’t cut back caspase-1 activation (Supplementary Fig. 5d, e). These findings counsel that NCF4, along with its position in driving ROS manufacturing, performs important roles downstream of ROS signaling resulting in inflammasome activation.
a FACS evaluation of ROS manufacturing in untreated (Media), LPS-treated (500 ng/mL), and LPS (500 ng/mL) plus ATP (5 mM)-treated WT and Ncf4–/– BMDMs for the indicated time. b Immunoblot evaluation of caspase-1 maturation and ELISA evaluation of IL-1β manufacturing (n = 4 biologically unbiased samples) in WT and Ncf4–/– BMDMs with out (Med) or with H2O2 remedy (10 μM, 1 h). c, d Co-IP evaluation of endogenous NCF4 interacting with ASC and NCF1 (c), and endogenous NCF1 interacting with ASC and NCF4 (d) in WT and Asc–/– BMDMs with out remedy (Med), handled with LPS (500 ng/mL, 4.5 h) or with LPS plus ATP (L+A; LPS, 500 ng/mL, 4 h and ATP, 5 mM, 15 min) for NLRP3 inflammasome activation. e, f Quantification evaluation of the relative portion of NCF4 (e) and NCF1 (f) interplay with different proteins in (c, d) (n = 2 biologically unbiased samples). g 3D-Confocal microscopy evaluation of the colocalization of NCF1 and NCF2, NCF1 and NCF4 in WT BMDMs stimulated with LPS (500 ng/mL, 4.5 h), or in WT, Ncf4–/–, and Asc–/– BMDMs stimulated with LPS (500 ng/mL, 4 h) plus ATP (5 mM, 30 min) for NLRP3 inflammasome activation. Scale bars: 10 μm. Knowledge are from 3 (b, IL-β) or consultant of three unbiased experiments with related outcomes for others. Knowledge symbolize Imply ± SEM for (b, IL-β), 2-sided Pupil’s t-test with out multiple-comparisons correction, p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
We speculated that membrane-bound NCF4 within the NADPH oxidase complicated was shifted to a cytoplasmic orientation throughout ASC speck formation and that this motion was related to elevated ROS depth. To additional examine the crucial roles of NCF4 in ASC speck formation, we carried out a co-IP evaluation to determine NCF4- and NCF1-interacting proteins in WT and Asc–/– BMDMs with and with out NLRP3 inflammasome activation. We discovered that, in WT BMDMs handled with LPS and ATP, NCF4 preferentially interacted with NCF1 and ASC, however interacted much less with NCF2 or NOX2 (Fig. 5c–f). In Asc–/– BMDMs, the interplay between NCF2 (or NOX2) and NCF4 (or NCF1) was considerably elevated in comparison with that in WT BMDMs after LPS and ATP remedy (Fig. 5c–f), suggesting that the inflammasome activation sign drives NCF4 (or NCF1) separation from NCF2 (or NOX2). These information point out that NCF1 and NCF4 separate from NCF2 and NOX2, the place NCF1 and NCF4 set up ASC-containing signaling hub driving inflammasome activation.
To verify the distinct intracellular localization of NCF1, NCF2, and NCF4 after LPS and LPS plus ATP therapies, we carried out a 3D structure evaluation of NCF1, NCF2, and NCF4 in WT, Ncf4–/–, and Asc–/– BMDMs. We discovered that NCF1, NCF2, and NCF4 colocalized to the membrane below LPS-treatment situations (Fig. 5g and Supplementary Film 1). Within the presence of LPS and ATP remedy, NCF1 and NCF4 preferentially colocalized nearer to the nucleus, whereas NCF2 localized largely to the plasma membrane. Compared, these three proteins colocalized to the membrane in Asc–/– BMDMs (Fig. 5g and Supplementary Film 1). Taken collectively, these outcomes point out that NCF4 and NCF1 type puncta to seed the spatial localization of ASC and promote ASC speck formation, whereas sustaining a supply of ROS manufacturing to gasoline activation of the creating inflammasome complicated.
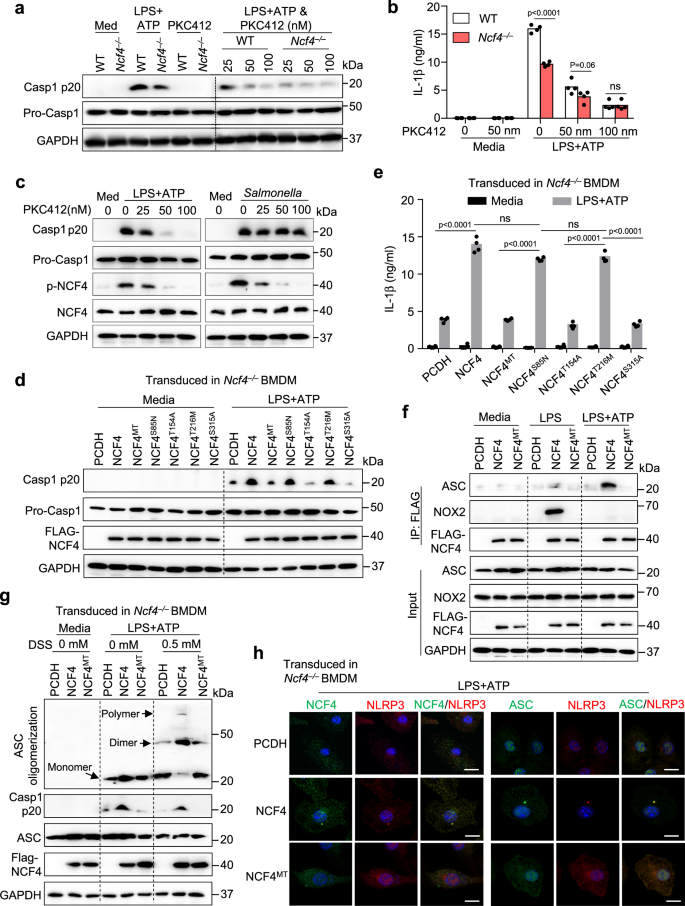
Phosphorylation of NCF4 is crucial for inflammasome activation
Phosphorylation of NCF4 is vital for NCF4 translocation to the plasma membrane and activation of NADPH oxidase39. To analyze the contribution of NCF4 phosphorylation to inflammasome activation, we handled WT and Ncf4–/– BMDMs with the PKC kinase inhibitor midostaurin (PKC412) at the side of LPS plus ATP or dsDNA transfection therapies to induce NLRP3 and AIM2 inflammasome activation, respectively. PKC412 inhibited, in a dose-dependent method, caspase-1 activation and IL-1β secretion in WT BMDMs following NLRP3 and AIM2 inflammasome activation (Fig. 6a, b and Supplementary Fig. 6a, b). As well as, remedy with growing concentrations of PKC412 correlated with diminished ranges of NCF4 phosphorylation and cell dying triggered by caspase-1 following NLRP3 inflammasome activation, however not following NLRC4 inflammasome activation (Fig. 6c and Supplementary Fig. 6c). To pinpoint the phosphorylation websites of NCF4 concerned in inflammasome activation, we constructed 4 single phosphorylation web site mutations (S85N, T154A, T216M, and S315A) and a quadruple mutation for all 4 phosphorylation websites (NCF4MT) (Supplementary Fig. 6d). We transduced WT NCF4 or its single or quadruple mutants into Ncf4–/– BMDMs and assessed NLRP3 inflammasome activation (Fig. 6d). Caspase-1 activation and IL-1β secretion in Ncf4–/– BMDMs have been rescued in WT NCF4-, NCF4S85N-, and NCF4T216M-transduced BMDMs, however not in NCF4MT-, NCF4T154A-, or NCF4S315A-transduced BMDMs (Fig. 6d, e). Remedy with LPS alone induced an interplay between WT NCF4 and NOX2, and remedy with LPS plus ATP induced an interplay between WT NCF4 and ASC (Fig. 6f). Nevertheless, these interactions have been inhibited in Ncf4–/– BMDMs transduced with NCF4MT (Fig. 6f).
a, b WT and Ncf4–/– BMDMs have been pretreated with PKC412 (25 nm, 50 nm, and 100 nm) for 1 h, and additional stimulated with LPS (500 ng/mL, 4 h) plus ATP (5 mM, 60 min) for NLRP3 inflammasome activation. Immunoblot evaluation of pro-caspase-1 (Professional-Casp1) and its subunit p20 (a), and evaluation of IL-1β launch (b, n = 4 biologically unbiased samples) in PKC412-treated and untreated BMDMs with and with out remedy for NLRP3 inflammasome activation. c Immunoblot evaluation of phosphorylation of NCF4, pro-caspase-1 (Professional-Casp1) and its subunit p20 in untreated and PKC412-treated WT BMDMs with and with out remedy with the NLRP3 activator LPS plus ATP (LPS, 500 ng/mL, 4 h and ATP, 5 mM, 60 min) or the NLRC4 activator Salmonella enterica Typhimurium (3 MOI, 2 h). d, e Immunoblot evaluation of pro-caspase-1 (Professional-Casp1) and its subunit p20 (d), and evaluation of IL-1β launch (e, n = 4 biologically unbiased samples) in Ncf4–/– BMDMs transduced with WT NCF4 and single or quadruple mutations (NCF4MT) of phosphorylation websites of NCF4 as indicated, additional stimulated with LPS plus ATP (LPS, 500 ng/mL, 4 h and ATP, 5 mM, 60 min). f Co-IP evaluation of transduced NCF4 interacting with ASC and NOX2 in Ncf4–/– BMDMs transduced with management plasmid (PCDH), WT NCF4 and quadruple mutations (NCF4MT) of phosphorylation websites of NCF4 with out remedy (Media), handled with LPS (500 ng/mL, 4.5 h) or with LPS plus ATP (LPS, 500 ng/mL, 4 h and ATP, 5 mM, 15 min). g ASC oligomerization evaluation in Ncf4–/– BMDMs transduced with management plasmid (PCDH), WT NCF4, and quadruple mutations (NCF4MT) of phosphorylation websites of NCF4 with out remedy (Media) and handled with LPS plus ATP (LPS, 500 ng/mL, 4 h and ATP, 5 mM, 30 min). h Confocal microscopy evaluation NCF4, NCF4MT, NLRP3, and ASC subcellular localization in Ncf4–/– BMDMs transduced with management plasmid (PCDH), WT NCF4 and quadruple mutations (NCF4MT) of phosphorylation websites of NCF4 response to LPS plus ATP (LPS, 500 ng/mL, 4 h and ATP, 5 mM, 30 min). Scale bars: 20 μm. Knowledge are from 3 (b, e) or consultant of three unbiased experiments with related outcomes (a, c, d, f–h). Knowledge symbolize Imply ± SEM for (b, e), 2-sided Pupil’s t-test with out multiple-comparisons correction, p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
As well as, ASC oligomerization was considerably diminished in Ncf4–/– BMDMs in contrast with WT BMDMs in response to LPS plus ATP (Supplementary Fig. 6e). Notably, Ncf4–/– BMDMs transduced with WT NCF4, however not NCF4MT, promoted ASC oligomerization in response to NLRP3 activation (Fig. 6g). Moreover, the puncta formation of NCF4 and NLRP3 or ASC in response to remedy with LPS alone or LPS plus ATP was inhibited in Ncf4–/– BMDMs transduced with NCF4MT, however not in Ncf4–/– BMDMs transduced with WT NCF4 (Fig. 6h and Supplementary Fig. 6f, g). These outcomes point out that the phosphorylation of NCF4 is crucial for NCF4 puncta seeding and NCF4-mediated inflammasome activation.
NCF4 is crucial for immunosurveillance within the early levels of colorectal most cancers development
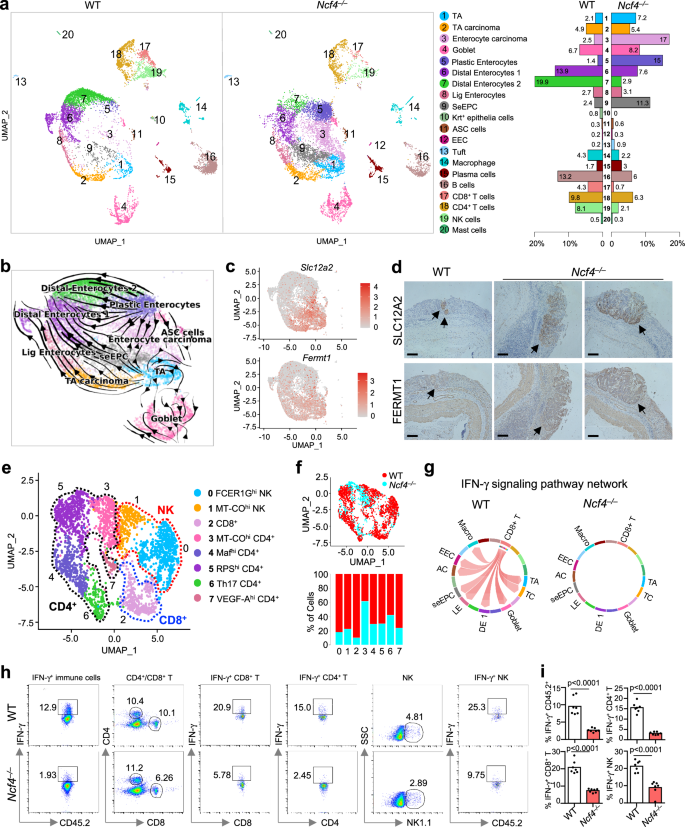
The tumor immune microenvironment (TIME) and tumor-infiltrating immune cells are pivotal in regulating tumor development and most cancers metastasis40. Though the TIME is a dynamic and heterogeneous community that has been a promising immunotherapeutic goal to struggle most cancers41, the position of inflammasomes in shaping this setting isn’t recognized. To characterize the mechanisms managed by the NCF4-inflammasome axis within the improvement of the microenvironment on the precancerous stage, we carried out single-cell RNA sequencing evaluation of colon tissues from WT and Ncf4–/– mice at Day 35 of the 70-day AOM-DSS mannequin (Supplementary Fig. 7a). After eliminating low-quality cells and doublets, a complete of 21,196 cells, together with 9127 and 12,069 cells, have been detected in WT and Ncf4–/– colons, respectively. These cells have been additional subjected to dimensionality discount and clustering evaluation (see “Strategies” part). To mitigate the impression ensuing from disparate cell counts between the 2 teams, a downsampling method was employed to make sure that cell counts within the Ncf4–/– colons have been equal to these within the WT colons. This step ensured comparability between the 2 teams for evaluation. Total, we annotated 20 clusters throughout the 2 compartments in line with the expression of marker genes for every cluster (Fig. 7a, Supplementary Fig. 7b, and Supplementary Knowledge 2). The 20 clusters comprised 13 epithelial cell subsets, together with transit-amplifying (TA) cells; a number of enterocyte-associated populations, adenoma-specific cells (ASCs); secretory clusters reminiscent of goblet cells, tuft cells, and enteroendocrine cells (EECs); and seven immune cell subsets (Fig. 7a). Two epithelial cell subsets, enterocyte carcinoma cells (Mki67, Slc12a2, Fermt1) and stem-early enterocyte precursor cells (SeEPCs; Reg3g, Hmgcs2, Slc12a2, Fermt1), exhibited stem-like options and have been considerably enriched in Ncf4–/– colons in comparison with WT colons (Fig. 7a). Notably, a definite cluster of epithelial cells characterised by Krt+ (Krt5, Krt6a, Krt13, Krt14) was absent within the Ncf4–/– colons, and the chances of two immune cell clusters, CD8+ T and NK cells, have been considerably diminished within the Ncf4–/– colons (Fig. 7a).
Single-cell RNA sequencing evaluation of colonic cells remoted from WT (n = 3) and Ncf4–/–mice (n = 3) at Day 35 publish AOM remedy. a Uniform manifold approximation and projection (UMAP) plot and fraction illustrating the distribution of immune and epithelial cells coloured by cluster. TA, transit-amplifying cells; Lig Enterocytes, giant gut gland enterocytes; ASC, adenoma-specific cells; EEC, enteroendocrine cells; and SeEPC, stem-early enterocyte precursor cells. b UMAP embedding of all epithelial cells with the stochastic illustration of the RNA velocity. c UMAP plot of epithelial cells exhibiting expression of a gene with stem-like options (tumor marker), Slc12a2 and Fermt1. d Consultant immunohistochemical picture of colon tissues from WT (n = 3) and Ncf4–/– mice (n = 3) stained with anti-SLC12A2 and anti-FERMT1. Scale bars: 10 μm. e UMAP embedding of sub-clustering of CD4+ T, CD8+ T, and NK cell clusters, coloured by subclusters. The identification of every cluster was redetermined by its predominant cells. The annotation of every subcluster was decided by its marker genes. f UMAP plot and fraction illustrating the distribution of the subcluster of immune cells in (e). The cells within the UMAP plot have been coloured by pattern identification. g Chord diagram exhibiting potential interactions or communication between completely different cell varieties mediated by IFN-γ signaling pathway. There was no vital interplay in Ncf4–/– mice in comparison with the WT mice. TA transit-amplifying cells, TC TA carcinoma, DE1 distal enterocytes 1, LE giant gut gland enterocytes, AC adenoma-specific cells, EEC enteroendocrine cells. h, i Consultant movement cytometry plots (h) and quantification evaluation (i) of IFN-γ+ cells in colonic CD45.2+, CD4+ T, CD8+ T and NK cells from WT (n = 7) and Ncf4–/– (n = 7) mice as indicated. Knowledge are consultant of three unbiased experiments with related outcomes (d, h, i). Knowledge symbolize Imply ± SEM for (i), 2-sided Pupil’s t-test with out multiple-comparisons correction, p-value is indicated within the graph. Supply information are supplied as a Supply Knowledge file.
An RNA velocity evaluation of all epithelial cells demonstrated a developmental trajectory in WT mice that was initiated by TA cells and concerned TA carcinoma and SeEPCs, and this trajectory bifurcated into giant gut gland (Lig) enterocytes and distal enterocyte cells. Nevertheless, NCF4 deficiency shifted the developmental trajectory from plastic enterocytes to enterocyte carcinoma cells (Fig. 7b). Furthermore, excessive ranges of stem-like transcription-related genes (Slc12a2, Fermt1, Ncl, Nop56 and Noxa1) have been detected in extremely proliferative cell clusters, such because the enterocyte carcinoma, SeEPC and TA clusters (Fig. 7c and Supplementary Fig. 7c). We additionally noticed an elevated expression of Slc12a2, Fermt1, Ncl, Nop56, and Noxa1 within the colon tissue of Ncf4–/– mice (Supplementary Fig. 7d). In people, the expression of SLC12A2, FERMT1, NCL, NOP56, and NOXA1 was considerably upregulated in human tumor cells in comparison with that in regular controls (Supplementary Fig. 7e). Immunohistochemical staining confirmed an elevated expression of SLC12A2 and FERMT1 within the colon tissue of Ncf4–/– mice on Day 35 (Fig. 7d). These outcomes point out that NCF4 defects trigger a considerable shift in enterocyte-associated clusters from mature enterocytes to plastic enterocytes, enterocyte carcinoma cells, and stem-like precursor cells, which can stop the event of Krt+ epithelial cells vital for wound therapeutic and keratinocyte differentiation (Supplementary Fig. 7f).
To chart the immune cell composition and modifications in cell states of WT and Ncf4–/– mice, we in contrast subpopulations of NK and T cells that play vital roles within the TIME and are activated by inflammasome-associated cytokines. We categorized NK and T cells into eight subclusters, together with two clusters of NK cells, one cluster of CD8+ T cells, and 5 clusters of CD4+ T cells, in line with the expression of particular genes (Fig. 7e, Supplementary Fig. 7g, h and Supplementary Knowledge 3). Notably, the chances of NK and CD8+ T cells have been considerably diminished within the Ncf4–/– colon in comparison with the WT colon (Fig. 7f). A circle plot highlighting the differential quantity and power of ligand–receptor (L–R) interactions between WT and Ncf4–/– colons indicated a profound discount in cell-cell interactions within the absence of NCF4 (Supplementary Fig. 7i). Moreover, a cell-cell interplay community evaluation revealed in depth interactions between CD8+ T cells and varied cell subsets mediated by IFN-γ signaling in WT however not in Ncf4–/– cells (Fig. 7g). According to the single-cell RNA sequencing outcomes, a FACS evaluation revealed that the proportions of NK and CD8+ T cells and IFN-γ-expressing cells have been considerably diminished within the colon tissue of Ncf4–/– mice in comparison with these in WT mice (Fig. 7h, i and Supplementary Fig. 7j). We additional verified a diminished manufacturing of IL-18, IL-1β and IFN-γ within the absence of NCF4 (Supplementary Fig. 7k). Constantly, absolutely the variety of IFN-γ-expressing NK cells, CD4+ T cells and CD8+ T cells was diminished within the colon tissue of Ncf4–/– mice in comparison with that in WT mice (Supplementary Fig. 8a). IL-18 injection remarkably rescued the discount in IFN-γ-expressing NK and CD8+ T cells within the colon tissue of Ncf4–/– mice handled with AOM-DSS (Supplementary Fig. 8b, c), and attenuated the epithelial dysplasia and tumorigenesis of Ncf4–/– mice handled with AOM-DSS (Supplementary Fig. 8d, e). Collectively, single-cell analyses revealed continuous cell transformation from regular to precancerous and cancerous states42, essential roles of IFN-γ signaling community in stopping this transformation progress, and NCF4 performs pivotal roles inhibiting this transformation by driving inflammasome-dependent activation of anti-tumor NK and CD8+ T cells throughout early levels of most cancers improvement (Supplementary Fig. 8f).