Lack of TRAF6 impairs leukemic cell operate in vitro and in vivo
To look at the function of TRAF6 in AML cells, we first in contrast the expression of TRAF6 in bone marrow (BM) cells from AML affected person samples with that of wholesome controls utilizing a publicly obtainable database [24]. RNA-seq evaluation revealed that the mRNA ranges of TRAF6 in AML had been larger in contrast with that in wholesome controls (Fig. 1A), suggesting the potential significance of TRAF6 in leukemogenesis. We beforehand demonstrated that the lack of TRAF6 in BM cells with a deficiency of Tet2, which is probably the most steadily noticed CHIP-associated mutation, leads to transformation to leukemia in mice [20]. Nonetheless, the function of TRAF6 in leukemia cells, quite than transformation from CHIP-associated mutants, stays unclear. We addressed this by flattening TRAF6 in varied human AML cell traces, together with HEL, TF-1, MV4;11, MOLM14, and THP-1, utilizing a doxycycline (DOX)-inducible shRNA system. TRAF6 knockdown notably inhibited proliferation in all examined cell traces besides THP-1 (Fig. 1B, C). To establish the premise for the lowered cell quantity in TRAF6-knockdown leukemia cells, we decided the impact of TRAF6 loss on apoptosis and cell cycle dynamics. Cell cycle evaluation revealed that the lack of TRAF6 led to an elevated proportion of cells within the G1 part and a decreased proportion within the S part amongst HEL, TF-1, MV4;11, and MOLM14 cells (Fig. 1D-E). Conversely, TRAF6 loss didn’t considerably affect apoptosis in these cells (knowledge not proven). These observations counsel that the inhibitory results of TRAF6 loss on leukemic cell proliferation stem from altered cell cycle development quite than from elevated apoptosis. To validate these findings in vivo, we established a myeloid leukemia mannequin induced by the leukemic fusion gene MLL-AF9 [22]. Remoted lineage− (Lin−) BM cells, derived from TRAF6+/+;MxCre and TRAF6flox/flox;MxCre mice [25], had been retrovirally transduced with vectors expressing MLL-AF9 (MSCV-IRES-GFP) after which serially replated in methylcellulose medium to pick reworked cells. The reworked BM cells (CD45.2+) had been transplanted into lethally-irradiated recipient mice together with wild-type BM cells (CD45.1+). At day 14 post-transplant, TRAF6 was deleted by intraperitoneally injecting polyinosinic-polycytidylic acid (pIpC) (Fig. 1F). As anticipated, the mice engrafted with MLL-AF9;Traf6+/+ cells developed a speedy and absolutely penetrant AML as much as 100 days post-transplantation (Fig. 1G). In distinction, mice engrafted with MLL-AF9;Traf6−/− cells developed a considerably delayed leukemia (Fig. 1G). In line with these findings, an immunophenotypic evaluation of peripheral blood revealed a lowered leukemic burden within the mice engrafted with MLL-AF9; Traf6−/− cells in contrast with these engrafted with MLL-AF9;Traf6+/+ cells on the time level (Fig. 1H). Cell cycle assessments in murine MLL-AF9 leukemic cells confirmed that TRAF6 loss impairs cell cycle development as noticed in human leukemic cells (Fig. 1I–J). Collectively, these findings affirm that TRAF6 loss suppresses leukemic cell operate each in vitro and in vivo.
A TRAF6 mRNA expression in wholesome BM CD34+ cells (n = 12) and AML (n = 451). The info for each wholesome BM CD34+ cells and AML sufferers had been retrieved from a broadcast database (BeatAML) [24]. B Immunoblot evaluation confirming knockdown of TRAF6 in leukemic cells upon addition of DOX(1 μg/mL). C Viable cell development of HEL, TF-1, MV4;11, MOLM14 and THP-1 cells transduced with the inducible shTRAF6 was assayed by trypan blue exclusion. The relative cell quantity was evaluated on 7 days after equal variety of the cells had been seeded. Information are introduced because the means ± SD from organic triplicates. Outcomes are consultant of three impartial assays. D Consultant move cytometric evaluation for the analysis of cell cycle of HEL cells transduced with inducible shTRAF6. E Share of cells in every cell cycle part, proven as means ± SD from organic replicates (n = 3). These outcomes are consultant of two impartial assays. F Overview of experimental design to look at the requirement of TRAF6 for the MLL-AF9 leukemic operate in vivo. Remoted Lin– BM cells had been transduced with retrovirus encoding MLL-AF9 and GFP. The transduced BM cells had been serially replated in methylcellulose medium to pick reworked cells. Lethally-irradiated recipient mice (CD45.2) had been engrafted with the reworked BM cells together with wild-type BM cells (CD45.1) for radioprotection. From day 14, the recipient mice acquired intraperitoneal injection of polyinosinic-polycytidylic acid [poly(I:C)] to delete TRAF6, after which had been monitored for engraftment and total survival. G Kaplan-Meier evaluation of total survival of mice engrafted with MLL-AF9;Traf6+/+ (n = 10) and MLL-AF9;Traf6−/− (n = 10) AML cells. H Abstract of the leukemic cell burden (GFP+) within the PB of the mice 10 weeks after transplant with MLL-AF9;Traf6+/+ (n = 8) and MLL-AF9;Traf6−/− (n = 10) AML cells. I Consultant flowcytometric profiles from EdU assay utilizing MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− AML cells. J Share of cells in every cell cycle part, proven as means ± SD for organic replicates (n = 6). HC, wholesome management. *P < 0.05; **P < 0.01, ***P < 0.001.
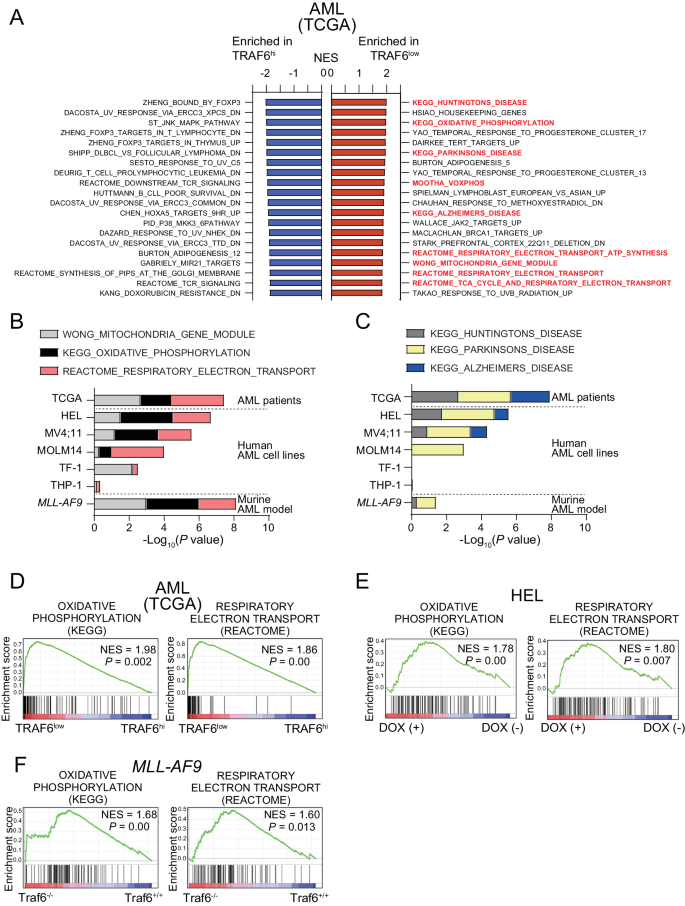
TRAF6 expression in AML is inversely correlated with mitochondria-related gene signatures
To find out the molecular mechanism of the inhibitory results of TRAF6 loss on leukemia operate, we stratified AML affected person samples primarily based on TRAF6 expression and in contrast the gene expression profiles between AML with excessive (Z rating >1.0, hereafter TRAF6hello AML) and low (Z rating <1.0, hereafter TRAF6low AML) TRAF6 expression. TRAF6 just isn’t solely a central mediator of innate immune signaling, however can be concerned in different immune features, resembling T cell receptor (TCR) signaling and immune management mediated by regulatory T cells [26, 27]. As anticipated, gene set enrichment evaluation revealed that immune-related gene units, resembling these related to FOXP3 targets, MAPK signaling, and TCR signaling, had been considerably enriched in TRAF6hello AML cells (Fig. 2A). Apparently, probably the most enriched gene units in TRAF6low AML cells included mitochondrial function-associated processes, resembling oxidative phosphorylation (OXPHOS), respiratory electron transport, and the TCA (tricarboxylic acid) cycle (Fig. 2A, B, D). Moreover, gene units associated to neurodegenerative illnesses, resembling Huntington’s, Parkinson’s, and Alzheimer’s illness, of which mitochondrial dysfunction performs a central function in pathogenesis, had been additionally overrepresented in TRAF6low AML cells (Fig. 2A, C) [28]. Equally, flattening TRAF6 in HEL, MV4;11, MOLM14, and TF-1 cells—however not in THP-1 cells—induced mitochondrial-related gene expression signatures (Fig. 2B, C, E). Notably, the absence of such enrichment in THP-1 cells aligns with our observations that TRAF6 loss didn’t affect their proliferation, suggesting a potential hyperlink between the mitochondrial gene expression profile and the proliferative capability affected by TRAF6 standing (Fig. 2B, C, E). This sample was additionally current in murine MLL-AF9;Traf6−/− leukemic cells (Fig. 2B, C, F). These outcomes counsel that TRAF6 loss in AML could also be linked to alterations within the expression of genes concerned in mitochondrial processes.
A Normalized enrichment scores (NES) from Gene Set Enrichment Evaluation (GSEA) for the highest 20 upregulated (pink, high) and downregulated (blue, backside), considerably altered gene units in TRAF6low in comparison with TRAF6hello AML affected person samples from the TCGA AML dataset [3]. High and low TRAF6 expressions outlined by: TRAF6low, Z rating <1; TRAF6hello, Z rating >1. B, C RNA sequencing evaluation of two teams: (1) human leukemic cell traces with inducible shTRAF6, handled with or with out doxycycline (DOX, 1 μg/mL) for 7 days, and (2) MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells. The mitochondrial states had been evaluated utilizing GSEA profiles primarily based on TRAF6low leukemic cells, with a concentrate on mitochondria-associated gene signatures organized by their P values (log10). Chosen gene set enrichment plots of AML sufferers stratified primarily based on low/excessive TRAF6 expression (D), HEL cells transduced with the inducible shTRAF6 (E), and MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells (F). NES normalized enrichment rating, DOX doxycyclin.
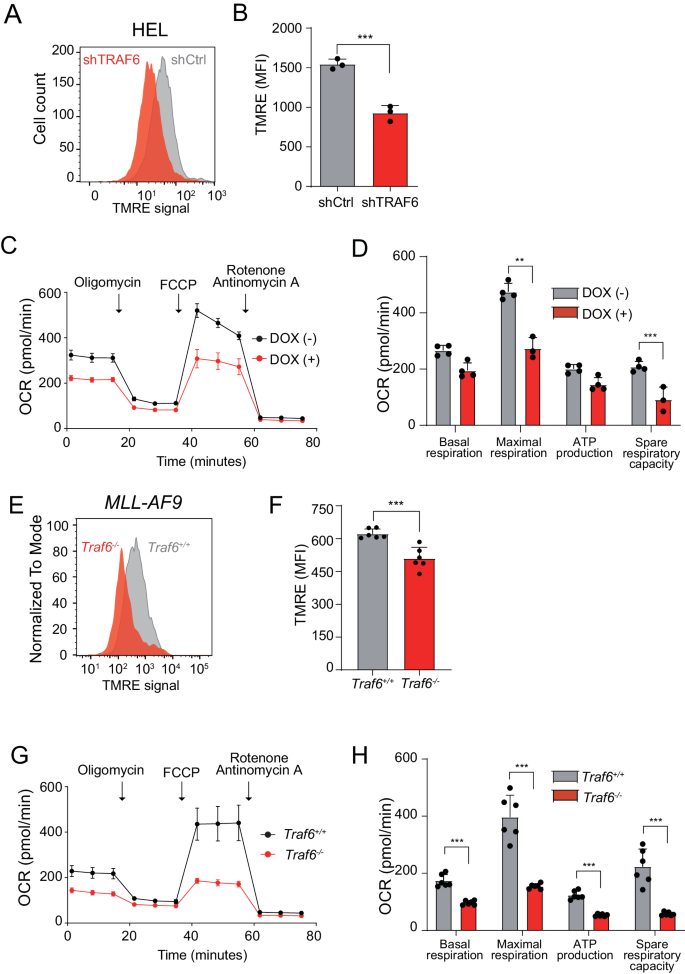
Lack of TRAF6 in AML leads to the perturbation of mitochondrial operate
Mitochondria are mobile organelles that generate power and metabolites required for cell survival and development. Vitality within the type of adenosine triphosphate (ATP) is primarily generated in mitochondria by the OXPHOS course of, wherein byproducts of the TCA cycle feed the electron transport chain (ETC) complexes and their electrons move by means of the ETC. Because the electrons are funneled by means of the varied complexes of the internal mitochondrial membrane, the ETC generates a mitochondrial membrane potential (MMP) that produces ATP [29]. To determine the affect of TRAF6 loss on mitochondrial operate in AML, we assessed MMP utilizing tetramethylrhodamine-ethyl ester dye in leukemia cells. Submit TRAF6 knockdown, HEL and TF-1 cells exhibited a discount in MMP in comparison with controls (Fig. 3A, B and Supplemental Fig. 1A). Additional analysis of mitochondrial operate with an extracellular flux analyzer indicated a lower in respiratory capability in TRAF6-knockdown HEL and TF-1 cells (Fig. 3C, D and Supplemental Fig. 1B). Conversely, MV4;11 and MOLM14 cells didn’t exhibit notable modifications in MMP or respiratory capability (Supplemental Fig. 1C–F). This lack of change means that these cell traces may compensate for mitochondrial operate disruption by means of the upregulation of mitochondrial genes, and that different molecular mechanisms could mitigate the consequences of TRAF6 knockdown on their proliferation. Equally, murine MLL-AF9;Traf6−/− leukemic cells displayed a decreased MMP and lowered mitochondrial respiratory capability in comparison with their Traf6+/+ counterparts (Fig. 3E–H). Collectively, these findings point out that TRAF6 loss can result in mitochondrial dysfunction in leukemic cells, contributing to the noticed phenotypic alterations in a subset of AML instances.
A Consultant move cytometry histograms of mitochondrial TMRE ranges in HEL cells expressing shTRAF6 or shControl (shCtrl). B Median fluorescent depth (MFI) of tetramethylrhodamine ethyl ester (TMRE) noticed from HEL cells expressing shTRAF6 or shCtrl. Information are introduced because the means ± SD from organic replicates (n = 3). Outcomes are consultant of two impartial assays. C Oxygen consumption fee (OCR) in HEL cells transduced with the inducible shTRAF6. Cells had been sequentially handled with oligomycin, fluoro-carbonyl cyanide phenylhydrazone (FCCP), and rotenone/antimycin A on the indicted time factors. Information are introduced because the means ± SD from technical replicates (n = 4). Outcomes are consultant of three impartial assays. D Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of HEL cells transduced with the inducible shTRAF6 calculated from the info of (C). Information are proven because the means ± SD (n = 4). E Consultant move cytometry histograms of mitochondrial TMRE ranges in MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells. F MFI of TMRE noticed from MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells. Information are introduced because the means ± SD from organic replicates (n = 6). Outcomes are consultant of two impartial assays. G OCR in MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells. Cells had been sequentially handled with oligomycin, FCCP, and rotenone/antimycin A on the indicted time factors. Information are introduced because the means ± SD from technical replicates (n = 6). Outcomes are consultant of two impartial assays. H Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells calculated from the info of (G). Information are proven because the means ± SD (n = 6). **, P < 0.01; ***, P < 0.001.
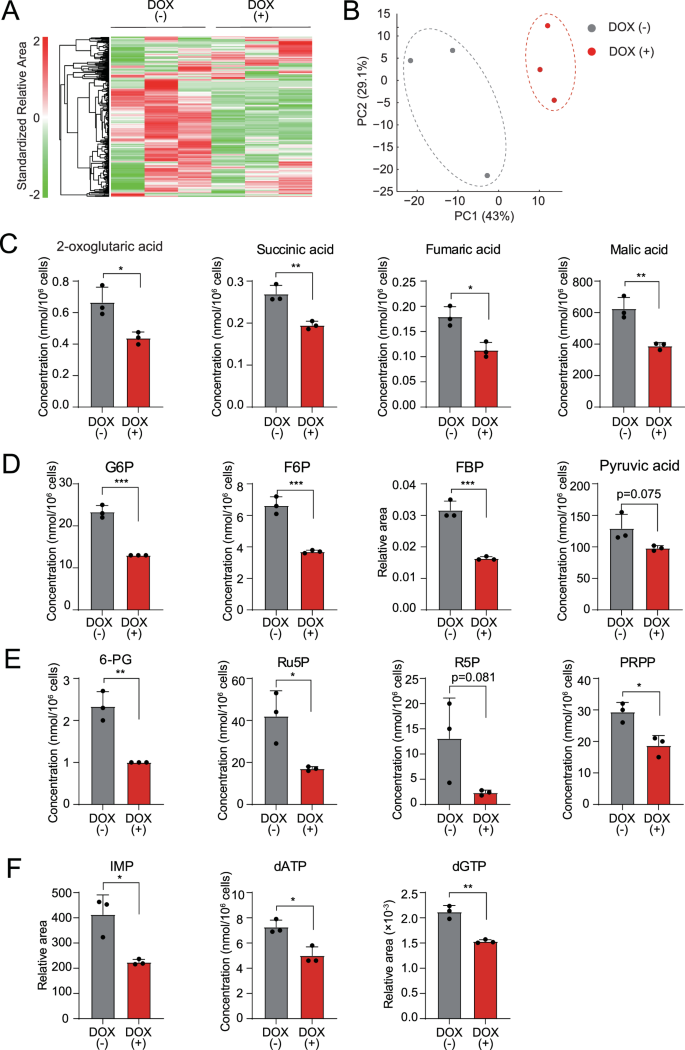
TRAF6 loss in AML offers rise to metabolic alterations
Mitochondria are important organelles that act as metabolic hubs and signaling platforms throughout the cell. Due to this fact, we evaluated the impact of TRAF6 loss on the metabolic profiles of leukemia cells by metabolome evaluation. Heatmap and principal element evaluation revealed that TRAF6 loss in HEL cells induced dynamic modifications in a number of metabolites (Fig. 4A, B). Since we noticed lowered MMP and impaired mitochondrial respiratory capability in TRAF6 knockdown leukemic cells (Fig. 3A–E), we first famous alterations in intermediates of the TCA cycle (Supplemental Fig. 2A). In line with our findings, the extent of a number of metabolites within the TCA cycle, together with 2-oxoglutaric acid, succinic acid, fumaric acid, and malic acid had been lowered in TRAF6 knockdown HEL cells (Fig. 4C). For power era by means of the TCA cycle, glucose within the cytoplasm finally breaks down into pyruvic acid, which is transported to the mitochondria and metabolized into acetyl-CoA below cardio situations (Supplemental Fig. 2A). The extent of most intermediates within the glycolytic pathway, together with glucose 6-phosphate (G6P), fructose 6-phosphate, fructose 1,6-diphosphate, and pyruvic acid had been lowered in TRAF6 knockdown HEL cells, suggesting a potential trigger for the discount of intermediates within the TCA cycle (Fig. 4D). Moreover, TRAF6 loss resulted within the discount of most intermediates within the pentose phosphate pathway (PPP), resembling phosphogluconic acid, ribulose 5-phosphate, ribose 5-phosphate, and phosphoribosyl pyrophosphate (Fig. 4E). As a result of PPP performs vital function in purine synthesis (Supplemental Fig. 2A), we examined the metabolites within the purine artificial pathway. Certainly, the extent of inosine monophosphate, deoxyadenosine triphosphate, and deoxyguanosine triphosphate, which acts as a precursor for nucleic acid synthesis throughout replication, was decreased upon TRAF6 loss (Fig. 4F). These reductions in a broad spectrum of metabolic intermediates led us to take a position that TRAF6 loss may suppress glucose uptake, thus affecting leukemic cell metabolism. Nonetheless, assessments of glucose uptake and intracellular glucose ranges in TRAF6-knockdown HEL and TF-1 cells revealed minimal modifications in these parameters (Supplemental Fig. 3A, B). Moreover, intracellular glucose degree in murine MLL-AF9;Traf6−/− leukemic cells was larger than in management cells (Supplemental Fig. 3A), suggesting that accumulation of unused intracellular glucose as a result of impairment of cascade reactions in metabolic pathways. Glucose uptake capability in murine MLL-AF9;Traf6−/− leukemic cells was suppressed in contrast with controls (Supplemental Fig. 3B), implying a damaging suggestions impact for prime intracellular glucose ranges. These findings point out that TRAF6 loss results in a worldwide discount in metabolic intermediates by means of mechanisms impartial of glucose uptake capability. Sometimes, mitochondrial dysfunction prompts enhanced glycolysis to compensate for lowered ATP manufacturing by impaired OXPHOS [30]. Nonetheless, the analysis of extracellular acidification fee (ECAR) in TRAF6-knockdown HEL and TF-1 cells, in addition to murine MLL-AF9;Traf6−/− leukemic cells, revealed the absence of such compensatory motion (Supplemental Fig. 3C). This means that the metabolic modifications induced by TRAF6 loss in leukemic cells are mediated by means of mechanisms affecting a broad vary of mobile processes. Since metabolic reprogramming is a trademark of malignancy and essential for supporting the heightened proliferation of tumor cells [31], our outcomes indicate that TRAF6 loss in leukemia cells induces metabolic alterations, contributing to their inhibited development capability.
A–F Metabolites in HEL cells transduced with the inducible shTRAF6 cultured for two days with or with out DOX (1 µg/mL) had been analyzed utilizing capillary electrophoresis Fourier rework mass spectrometry (CE-FTMS) (n = 3). Hierarchical clustering heatmap evaluation (A) and principal element evaluation (B) of all metabolites that had been detected as a peak by CE-FTMS (n = 483). The concentrations or relative space of chosen metabolites in TCA cycle (C), glycolytic pathway (D), pentose phosphate pathway (E) and purine synthesis pathway (F). G6P glucose 6-phosphate, F6P fructose 6-phosphate, FBP fructose 1,6-diphosphate, 6-PG 6-phosphogluconic acid, Ru5P ribulose 5-phosphate, R5P ribose 5-phosphate, PRPP phosphoribosyl pyrophosphate, IMP inosine monophosphate, dATP deoxyadenosine triphosphate, dGTP deoxyguanosine triphosphate. *P < 0.05; **, <0.01; ***P < 0.001.
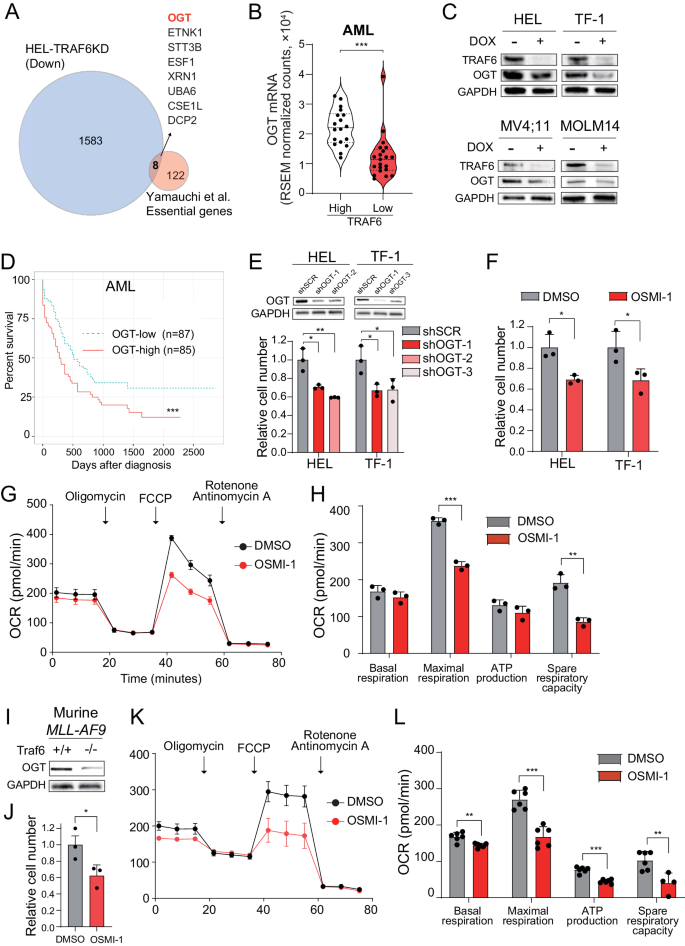
OGT is a possible mediator of metabolic reprogramming regulated by TRAF6 in leukemia
To establish the mechanism of metabolic alterations pushed by TRAF6 loss in leukemia, we in contrast the upregulated genes in TRAF6-knockdown HEL cells (1.5-fold, P < 0.05) (Supplemental Desk 1) with the 130 beforehand recognized important genes for AML cell survival in vitro and in vivo [32]. We discovered that 8 genes overlapped, and O-GlcNAc transferase (OGT) and ETNK1 had been related to mitochondrial operate and leukemia cell survival (Fig. 5A) [33, 34]. Stratification of AML sufferers revealed that TRAF6 gene expression in human AML affected person samples was positively correlated with the expression of OGT, however not ETNK1 (Fig. 5B and knowledge not proven). Correspondingly, TRAF6 knockdown in HEL and TF-1 cells additionally led to lowered OGT protein ranges (Fig. 5C). Intriguingly, in MV4;11 and MOLM14 cells, the place TRAF6 loss didn’t considerably have an effect on mitochondrial operate (Supplemental Fig. 1C–F), OGT expression was additionally suppressed (Fig. 5C), suggesting a broader constructive correlation between TRAF6 and OGT expression in leukemic cells. Moreover, stratification of AML sufferers revealed that OGT-low sufferers had been related to longer total survival (P < 0.001) (Fig. 5D). This led us to analyze OGT as a mediator of TRAF6-regulated mitochondrial operate in leukemia cells. To guage the function of OGT in leukemia development, we examined the affect of OGT loss or inhibition on leukemic cell development. Inducing OGT-targeted shRNAs in HEL and TF-1 cells considerably lowered cell numbers (Fig. 5E). Moreover, remedy with the OGT inhibitor OSMI-1 suppressed cell development within the leukemic cells, correlating with diminished mitochondrial respiratory operate (Fig. 5F–H). Comparable tendencies had been noticed in murine MLL-AF9 leukemic cells, exhibiting a constructive correlation between Traf6 and Ogt ranges, and susceptibility to OSMI-1, together with modifications in mitochondrial respiratory operate (Fig. 5I–L). These findings counsel that OGT loss mimics TRAF6 loss results on leukemia cell metabolism and development capability.
A Venn diagram of downregulated genes (1.5-fold, P < 0.05) in DOX-treated HEL cells transduced with the inducible shTRAF6 (relative to untreated HEL cells transduced with the inducible shTRAF6) and 130 AML important genes recognized by CRISPR-Cas9 screens [32]. B OGT mRNA expression in AML sufferers stratified on TRAF6 expression (TRAF6hello, n = 18; TRAF6low, n = 21) [3]. C Immunoblot evaluation of TRAF6 and OGT in HEL, TF-1, MV4;11 and MOLM14 cells transduced with the inducible shTRAF6, handled with or with out DOX (1 μg/mL) for 3 days. D Total survival of AML sufferers stratified on OGT expression (OGThello, n = 85; OGTlow, n = 87) [3]. Excessive and low OGT expressions outlined by: OGThello, above median; OGTlow, beneath median. Survival curves had been generated utilizing the BloodSpot database (https://www.fobinf.com/). E Immunoblot evaluation of OGT in HEL and TF-1 expressing shOGT or shSCR (Higher panel). Viable cell variety of HEL and TF-1 expressing shOGT or shSCR was assayed by trypan blue exclusion. The relative cell quantity was evaluated 72 h after equal variety of the cells had been seeded. Information are introduced because the means ± SD from technical triplicates. Outcomes are consultant of two impartial assays. F Viable cell variety of HEL and TF-1 uncovered to twenty µM of OSMI-1 (OGT-inhibitor) was assayed by trypan blue exclusion. The relative cell quantity was evaluated 72 h after equal variety of the cells had been seeded. Information are introduced because the means ± SD from technical triplicates. Outcomes are consultant of two impartial assays. G OCR in HEL cells handled with OSMI-1 (20 μM) for 48 h. Cells had been sequentially handled with oligomycin. FCCP, and rotenone/antimycin A on the indicted time factors. Information are introduced because the means ± SD from technical triplicates. Outcomes are consultant of two impartial assays. H Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of HEL cells handled with OSMI-1 calculated from the info of (G). Information are introduced because the means ± SD (n = 3). I Immunoblot evaluation of OGT in MLL-AF9;Traf6+/+ and MLL-AF9;Traf6−/− leukemic cells. J Viable cell variety of MLL-AF9 leukemic cells uncovered to 2 µM of OSMI-1 was assayed by trypan blue exclusion. The relative cell quantity was evaluated 72 h after equal variety of the cells had been seeded. Information are introduced because the means ± SD from technical triplicates. Outcomes are consultant of two impartial assays. Ok MLL-AF9 leukemic cells (2μM) for 48h. Cells had been sequentially handled with oligomycin. FCCP, and rotenone/antimycin A on the indicted time factors. Information are introduced because the means ± SD from technical replicates (n = 6). Outcomes are consultant of two impartial assays. L Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of MLL-AF9 leukemic cells handled with OSMI-1 calculated from the info of (Ok). Information are introduced because the means ± SD (n = 6).*P < 0.05; **<0.01; ***P < 0.001.
O-GlcNAc modification is a possible contributor to TRAF6-mediated metabolic reprogramming and leukemia development
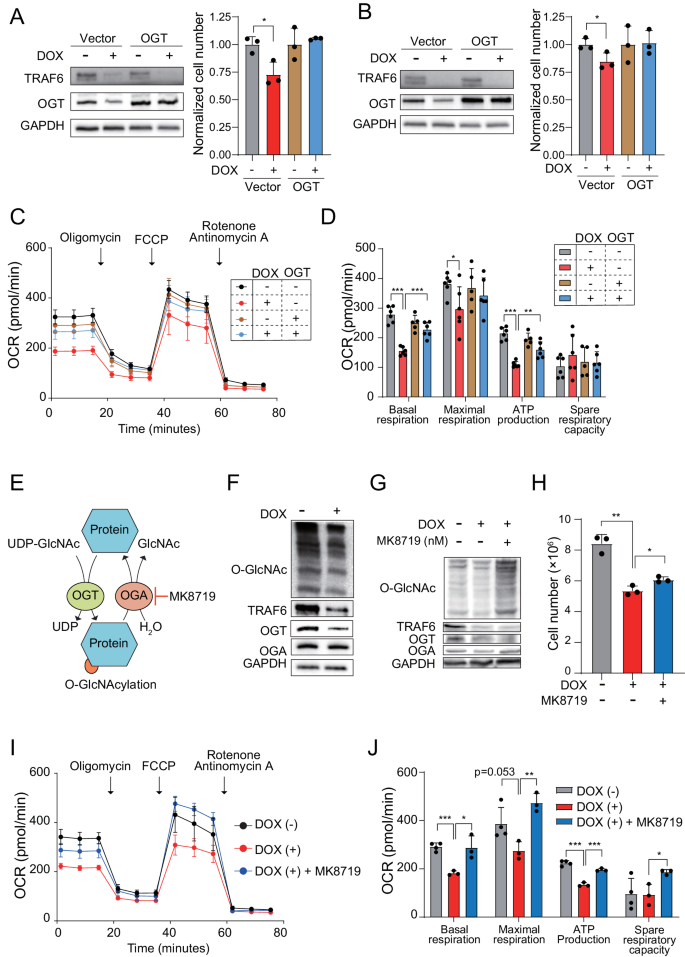
To analyze OGT’s function within the regulation of mitochondrial operate mediated by TRAF6 in leukemia, we utilized a lentiviral system to overexpress OGT in TRAF6 knockdown human leukemic cells, inspecting its affect on development capability. The pressured overexpression of OGT efficiently counteracted inhibitory impact of TRAF6 loss on the proliferation defect in HEL and TF-1, together with a corresponding restoration in mitochondrial respiratory capability (Fig. 6A–D). This outcome suggests a hyperlink between OGT and the metabolic dysregulation resulting from TRAF6 loss, affecting leukemic cell proliferation. Nonetheless, in murine MLL-AF9;Traf6−/− leukemic cells, OGT overexpression didn’t rectify the proliferation defect (Supplemental Fig. 4A). This statement aligns with earlier analysis indicating that each extreme and inadequate ranges of OGT can negatively affect cell metabolism and proliferation [35].
Immunoblot evaluation of OGT in HEL (A) and TF-1 (B) transduced with inducible shTRAF6 expressing both management vector or cDNA of OGT, cultured with or with out DOX (1 μg/mL) for 3 days (left panel). Viable cell development of the cells was assayed by trypan blue exclusion (proper panel). The normalized cell rely, relative to untreated cells, was decided 72 h post-seeding of an equal variety of cells. Information are introduced because the means ± SD from technical triplicates. Outcomes are consultant of two impartial assays. C OCR in HEL cells transduced with inducible shTRAF6, expressing management vector or cDNA of OGT, untreated or handled with DOX for 3 days. Cells had been sequentially handled with oligomycin. FCCP, and rotenone/antimycin A on the indicted time factors. Information are proven because the means ± SD for technical replicate analyses (n = 6). Outcomes are consultant of two impartial assays. D Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of HEL cells calculated from the info of (C). The info are proven because the means ± SD (n = 6). E Schematic of O-GlcNacylation. F Immunoblotting of HEL cells transduced with the inducible shTRAF6, handled with or with out DOX (1 μg/mL) for 3 days. G Immunoblotting of HEL cells transduced with the inducible shTRAF6, untreated with DOX, handled with DOX, and handled with DOX and 100 nM of MK8719 (OGA inhibitor). H 100 thousand HEL cells transduced with inducible shTRAF6 had been cultured with 1 μM of MK8719 for 7 days. Viable cell development of the cells was assayed by trypan blue exclusion. Information are introduced because the means ± SD for technical triplicates. Outcomes are consultant of two impartial assays. I OCR in HEL cells transduced with the inducible shTRAF6 untreated with DOX, handled with DOX (1 μg/mL), and handled with DOX (1 μg/mL) and 200 nM of MK8719 (OGA inhibitor). Cells had been sequentially handled with oligomycin. FCCP, and rotenone/antimycin A on the indicted time factors. Information are introduced because the means ± SD from technical replicate analyses (n = 3–4). Outcomes are consultant of two impartial assays. J Basal respiration, maximal respiration, ATP manufacturing and spare respiratory capacities of HEL cells transduced with the inducible shTRAF6 calculated from the info of (I). Information are proven because the means ± SD (n = 3–4). *P < 0.05; **<0.01; ***P < 0.001.
OGT catalyzes the addition of an O-GlcNAc moiety to serine or threonine residues of protein substrates, whereas O-GlcNAcase (OGA) removes these modifications (Fig. 6E) [36]. Notably, the extent of O-GlcNAc modification was lowered in TRAF6-knockdown HEL and TF-1 cells, in addition to in murine MLL-AF9;Traf6−/− leukemic cells (Fig. 6F and Supplemental Fig. 4B). Provided that quite a few metabolic enzymes are O-GlcNAc targets [36, 37], we hypothesized that the discount on this modification disrupts metabolic reprogramming attributable to TRAF6 loss in leukemia. To check this speculation, we examined the consequences of the OGA inhibitor MK8719 on the expansion capability of leukemia cells impacted by TRAF6 loss. MK8719 remedy in TRAF6-knockdown HEL cells and murine MLL-AF9;Traf6–/– leukemic cells restored O-GlcNAc ranges (Fig. 6G and Supplemental Fig. 4C) and inhibited the consequences of TRAF6 loss on development capability, correlating with a restoration in mitochondrial respiratory operate (Fig. 6H–J and Supplemental Fig. 4D-F). Nonetheless, MK8719 didn’t right the proliferation defect in TRAF6-knockdown TF-1 cells (knowledge not proven). Given {that a} broad vary of metabolic enzymes are targets of O-GlcNAcylation, the metabolic standing in leukemia cells is probably going finely regulated by the stability between OGT and OGA expression and exercise.
Taken collectively, these outcomes counsel that the fine-tuned and sophisticated regulation of O-GlcNAc modification performs an influential function in TRAF6-mediated metabolic reprogramming of leukemic cells.