Improvement of JMF4073 as an inhibitor of TMPK and CMPK
The survival and progress of most cancers cells require a enough provide of the 4 dNTPs for DNA restore and replication. Thymidylate kinase (TMPK) and cytidylate kinase (CMPK) are important for the biosynthesis of TTP and the salvage synthesis of dCTP, respectively (Fig. 1a). 5-Fluorouracil (5-FU), which irreversibly inhibits thymidylate synthase, has been extensively used for chemotherapy. Nonetheless, 5-FU exerts normal toxicity on regular proliferating cells as a consequence of 5-FdUTP and dUTP misincorporation into DNA and 5-FUTP into RNA24. Now we have beforehand recognized a TMPK inhibitor, particularly YMU1. Utilizing YMU1 as a lead, the compounds JMF2977 and JMF4073 had been synthesized. The IC50 of YMU1, JMF2977, and JMF4073 was 0.8, 0.5, and 0.16 μM, respectively. JMF4073, containing a fluoride substitution on the C-position of the pyridine ring, has the bottom IC50 worth and better solubility, as indicated by the clogP worth (Fig. 1b). By molecular simulation, we have now beforehand proven that YMU1 acts on the catalytic pocket of TMPK, which causes the expulsion of the lid area to offer an open conformation that inhibits the catalytic course of19. Since CMPK additionally has a lid area25, we then examined whether or not JMF4073 is an inhibitor of CMPK. The outcomes confirmed that JMF4073 inhibits each TMPK and CMPK at an identical IC50, 0.17 μM, whereas YMU1 is much less potent to CMPK (Fig. 1b). Thus, JMF4073 is certainly an inhibitor of TMPK and CMPK. In accordance, therapy of mouse 32D myeloid progenitor and human HEK-293T cells with JMF4073 for six h considerably diminished the mobile degree of TTP and dCTP (Fig. 1c).
a Schematic view of the synthesis of dTTP and dCTP. TMPK and CMPK mediate the synthesis of dTDP and dCDP, respectively, which subsequently are transformed to dTTP and dCTP by nucleoside diphosphate kinase (NDPK). Thymidylate synthase (TS) converts dUMP to dTMP, which is inhibited by 5-FU. Ribonucleotide reductase (RNR) mediates the de novo synthesis of dCDP, dADP, dUDP, and dGDP. Salvage synthesis of dTMP and dCMP is mediated by thymidine kinase and deoxycytidine kinase. b The chemical buildings, clogP, and IC50 of YMU1, JMF2977, and JMF4073. The clogP values had been calculated by Chemsketch. The IC50 values in opposition to hTMPK and hCMPK had been measured by NADH-coupled TMPK assay. c The measurement of dTTP and dCTP swimming pools in untransformed 32D and HEK-293T cells after incubation with JMF4073 (10 μM) for six h. Information are represented as means ± S.D., n = 3 organic replicates. Asterisks denote *p < 0.05, **p < 0.01, or ***p < 0.001, from unpaired two-tailed Pupil’s t-test.
We then decided the mode of JMF4073 inhibition by pre-incubating purified TMPK or CMPK protein with numerous concentrations of JMF4073 and measured the alteration in Vmax and Km by NADH-coupled enzymatic assay. The kinetic information demonstrated that JMF4073 inhibits TMPK and CMPK in a combined inhibitory mode as indicated by the elevated Km for the substrates with decreased Vmax. The inhibition fixed (Ki) of JMF4073 within the dTMP and ATP kinetic evaluation of TMPK was 0.16 μM and 0.005 μM, respectively. The Ki within the CMP and ATP kinetic evaluation of CMPK was 0.37 μM and 0.06 μM, respectively (Desk 1). These kinetic analyses point out that JMF4073 has the next affinity to the ATP binding websites in TMPK and CMPK.
JMF4073 eliminates WT-Bcr-Abl-transformed myeloid cells in vitro and in vivo
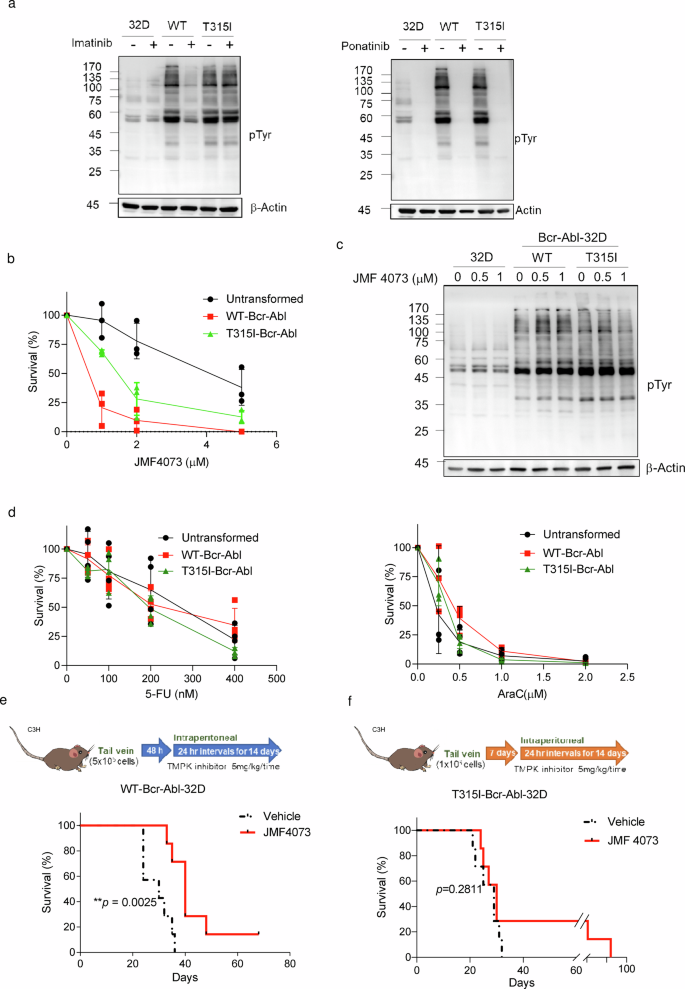
Because the balanced provide of dNTPs is crucial for overcoming DNA injury and replication stress for cell survival, we then examined whether or not JMF4073 therapy may selectively suppress the expansion of Bcr-Abl-transformed however not untransformed myeloid cells. To this finish, 32D cells, a mouse myeloid progenitor line which proliferation depends on interleukin-3 (IL-3)26, had been employed for WT- and T315I-Bcr-Abl transformation. The proliferation of those reworked cells was unbiased of IL-326. By analyzing quite a lot of Bcr-Abl downstream alerts together with pAkt-S473, pS6K-T389 within the PI3K/Akt/mTOR pathway, and pStat5-Y694, we confirmed these alerts equally elevated in these two totally different reworked cells. As anticipated, these alerts had been delicate to imatinib therapy solely in WT-Bcr-Abl-32D cells however not T315I-Bcr-Abl-32D cells, whereas ponatinib27 therapy abolished these alerts in each cell strains (Supplementary Fig. 1). It was famous that WT- and T315I-Bcr-Abl transformation markedly stimulated the extent of general tyrosine phosphorylation, which was diminished by ponatinib therapy. Nonetheless, not like imatinib therapy, the basal degree of tyrosine phosphorylation in 32D progenitor cells was additionally diminished by ponatinib, indicating its non-specific inhibitory impact (Fig. 2a).
a The comparability of general pTyr in unstranformed, WT, and T315I Bcr-Abl -transformed 32D myeloid progenitor cells, After the therapy of imatinib (2 μM) and ponatinib (2 μM) for 4 h, cell lysates had been analyzed by Western blot utilizing phosphotyrosine (pY99) antibody. Cells had been handled with JMF4073 on the indicated concentrations for (b) viability assays and (c) Western blot of tyrosine phosphorylation. d Cells had been handled with 5-FU, and ara-C on the indicated concentrations for viability assays. Information are represented as means ± S.D., n = 3 organic replicates. e C3H/HeNCrNarl mice had been intravenously injected with 5 × 105 cells of WT-Bcr-Abl-32D cell. After 48 h of transplantation, mice had been handled with car (n = 7), or JMF4073 (5 mg/kg/time, n = 7) by intraperitoneal injection at 24 h intervals for 14 days. f C3H/HeNCrNarl mice had been intravenously injected with 1 × 106 cells of T315I-Bcr-Abl-32D cell. After 7 days of transplantation, mice had been handled with car (n = 7), or JMF4073 (5 mg/kg/time, n = 7) by intraperitoneal injection at 24 h intervals for 14 days. The Kaplan–Meier plot reveals the survival of mice with therapy as indicated. Asterisks denote *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001 from unpaired two-tailed Pupil’s t-test or Log-rank (Mantel–Cox) check. The mice photos are hand-drawn utilizing the free Samsung PENUP software program.
Subsequent, JMF4073 sensitivity in non-transformed, WT-, and T315I-Bcr-Abl-transformed 32D cells was in contrast. The outcomes confirmed that WT-Bcr-Abl cells had been extremely vulnerable to JMF4073 with GI50 0.67 μM as in comparison with 1.5 μM and 4.2 μM for T315I-Bcr-Abl-32D and untransformed 32D cells, respectively (Fig. 2b). The degrees of general tyrosine phosphorylation in these cells had been unaffected by JMF4073 therapy (Fig. 2c). 5-FU and cytosine arabinoside (ara-C) are nucleoside analog medication extensively used to induce DNA and RNA toxicity for chemotherapy28,29. The sensitivity of 5-FU and ara-C had been related in WT-, T315I-Bcr-Abl-32D and untransformed 32D progenitor cells (Fig. second). Thus, JMF4073 therapy has a bonus over 5-FU and ara-C in selectively suppressing Bcr-Abl-transformed however not untransformed myeloid progenitor cells.
We additional examined the in vivo therapeutic impact of JMF4073 in WT-Bcr-Abl-CML mice. WT-Bcr-Abl-transformed 32D cells had been transplanted into C3H/HeNCrNarl mice. After 48 h, these mice had been handled with a day by day intraperitoneal injection of JMF4073 for 14 days (Fig. 2e). After the remedy, the hematology evaluation confirmed white blood counts had been introduced down by JMF4073 therapy in WT-Bcr-Abl-32D bearing mice (Supplementary Desk 1). Furthermore, therapy with JMF4073 for 14 days extended the survival of leukemia mice (Fig. 2f). Since T315I-Bcr-Abl-transformed 32D cells took longer time to develop leukemia phenotype than WT Bcr-Abl cells, we transplanted 2-fold extra cells into C3H/HeNCrNarl mice. After transplantation for 7 days, these mice had been handled with JMF4073 for 14 days. Not like the WT-Bcr-Abl mice, the survival of T315I leukemia mice was not considerably extended by JMF4073 (Fig. 2g; Supplementary Desk 2). Thus, not like WT-Bcr-Abl cells, T315I-Bcr-Abl cells are much less susceptible to JMF4073 in vitro and in vivo.
T315I-Bcr-Abl-32D cells have excessive dTTP pool and low replication stress with elevated GSH biosynthesis
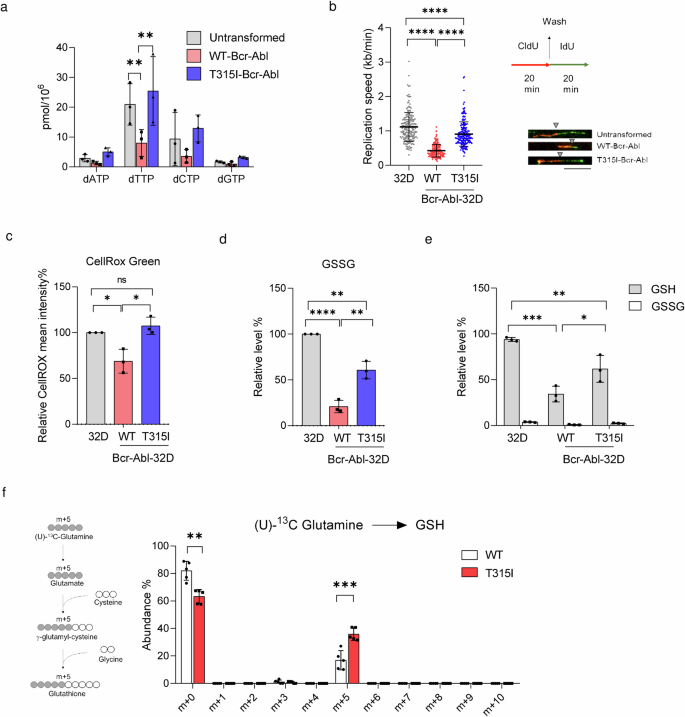
To know the underlying mechanism for the differential susceptibility to JMF4073 in these cells, we carried out quantitative evaluation of 4 dNTPs. Information confirmed that WT-Bcr-Abl-32D cells had very low ranges of dNTPs as in comparison with these in T315I-Bcr-Abl-32D and untransformed 32D cells, with probably the most important distinction in dTTP pool (Fig. 3a). Compelling proof has proven that oncogene-induced replication stress and dNTP exhaustion30,31. Since nucleotide provide performs a crucial function in DNA replication, we additional carried out DNA fiber assay by measuring the lengths of chase IdU-labeled DNA replication tracks that hyperlink to pulse CldU-labeling, which is a gold normal for assessing replication stress32. The evaluation confirmed that the replication observe lengths in WT-Bcr-Abl cells had been clearly a lot shorter as in comparison with these in 32D cells (Fig. 3b). Intriguingly, the replication observe lengths had been fairly related in T315I-Bcr-Abl-32D and 32D cells. These outcomes evoke the hypothesis that larger dTTP degree in T315I-Bcr-Abl-32D cells may forestall DNA replication stress to have an effect on JMF4073 sensitivity.
Untransformed, WT-, and T315I-Bcr-Abl reworked 32D cells had been subjected to (a) the measurement of 4 dNTP ranges, b DNA fiber evaluation to find out the replication fork pace in DNA replication pace (n = 200 fibers for every cell line) (Left). The workflow of DNA fiber labeling (Proper). The consultant labeled fibers are proven beneath. Replication pace in kb/min was calculated by the measured size of IdU (inexperienced) linking to CldU (pink) in μm with a conversion issue of 0.34 μm/kb divided by the length of the labeling pulse (Scale bar = 10 μm). The grey triangle signifies the boundary between pink fluorescence and inexperienced fluorescence. c The measurement of intracellular ROS by the fluorescence depth of CellROX Inexperienced staining utilizing stream cytometric evaluation. Information are introduced as imply depth relative to untransformed 32D cells. d, e The measurement of GSSG and GSH. Information are introduced relative to untransformed 32D cells. f WT- and T315I-Bcr-Abl-32D cells after incubation with 4 mM U-13C glutamine medium for two h. Schematic illustration of the metabolic path U-13C glutamine incorporation into the GSH (left). Grey and white circles point out 13C-label, and unlabeled 12C, respectively. The traced isotopologue abundance of GSH is proven (proper). Information are represented as means ± S.D., n = 3 organic replicates. Asterisks denote *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001 from unpaired two-tailed Pupil’s t-test.
Since DNA replication stress may also be regulated by the redox standing, we then measured ROS, GSSG, and diminished GSH in these cells. The outcomes confirmed that the entire ranges of ROS and GSSG had been larger in T315I-Bcr-Abl-32D than these in WT-Bcr-Abl-32D cells (Fig. 3c, d). Regardless of these variations, the entire quantity of diminished glutathione (GSH) in WT-Bcr-Abl-32D cells was considerably decrease than that in untransformed and T315I-Bcr-Abl-32D cells (Fig. 3e). We additional in contrast the biosynthesis GSH synthesis by flux evaluation in WT- and T315I-Bcr-Abl-32D cells incubated with (U)-13C-glutamine. The evaluation revealed a big enhance in the m + 5 GSH degree in T315I-Bcr-Abl cells as in comparison with that in WT-Bcr-Abl cells (Fig. 3f), suggesting that upregulation of GSH biosynthesis might need a performance in counteracting the rise in ROS. General, our observations indicate the relevance of JMF4073 sensitivity to excessive replication stress related to low dTTP/GSH ranges in WT-Bcr-Abl-32D cells.
ATF4 activation upregulates GSH and dTTP swimming pools in T315I-Bcr-Abl-32D cells
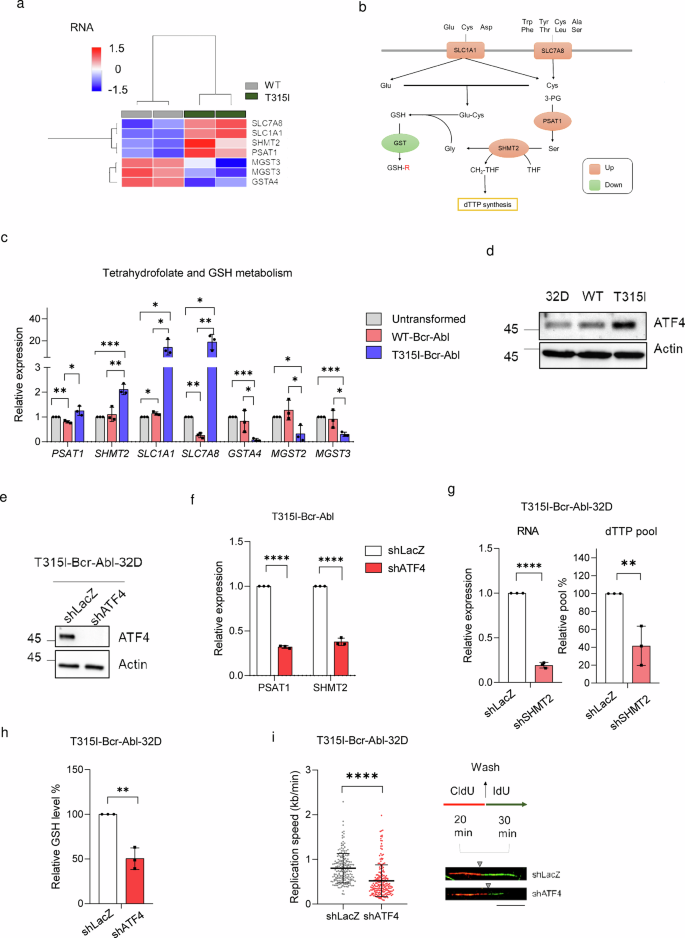
Subsequent, we wish to deal with the questions of what causes the will increase in dTTP and GSH biosynthesis in T315I-Bcr-Abl and whether or not blocking the upregulation of those two components can enhance JMF4073 sensitivity. Of notice, the evaluation by western blot revealed that the degrees of enzymes concerned in dTTP biosynthesis, like R1 and R2 subunits of ribonucleotide reductase (RNR), thymidylate synthase (TS), thymidylate kinase (TMPK), and thymidine kinase 1 (TK1), had been related in WT and T315I-Bcr-Abl cells (Supplementary Fig. 2). To comprehensively perceive the gene expression modifications answerable for the variations in dTTP and GSH biosynthesis, we carried out RNA sequencing evaluation of WT- and T315I-Bcr-Abl-32D cells. The gene set enrichment evaluation (GSEA) of gene ontology (GO) pathways of RNA sequencing information revealed that T315I-Bcr-Abl cells have a rise in ATF4-mediated built-in stress response (ISR) signaling (Supplementary Fig. 3), which is understood to upregulate the expression of genes concerned in amino acid transport or synthesis, one-carbon metabolism, and glutathione biosynthesis33,34 (Fig. 4a, b). RT-qPCR analyses validated the upregulation of ATF4 goal genes, together with SLC1A1, SLC7A8, phosphoserine aminotransferase 1 (PSAT1), and serine hydroxymethyltransferase 2 (SHMT2) in T315I-Bcr-Abl cells (Fig. 4c). In the meantime, the expression ranges of numerous GSH transferase genes had been downregulated in T315I-Bcr-Abl-32D cells. The Western blot evaluation confirmed that T315I-Bcr-Abl-32D cells clearly expressed larger degree of ATF4 (Fig. 4d). Notably, SHMT2 is a mitochondrial enzyme contributory to one-carbon metabolism by changing serine and tetrahydrofolate (THF) into glycine for GSH synthesis and a one-carbon unit, 5,10-methylenetetrahydrofolate (5,10-CH2-THF), for dTTP synthesis35, respectively. Knockdown of ATF4 by lentiviral shRNA an infection diminished RNA ranges of PSAT1 and SHMT2 in T315I-Bcr-Abl-32D cells (Fig. 4e, f). Knockdown of SHMT2 in T315-Bcr-Abl-32D cells led to about 50% discount in dTTP pool in these cells (Fig. 4g). Subsequently, ATF4-mediated upregulation of SHMT2 contributes to the next degree of dTTP in T315I-Bcr-Abl-32D cells.
a The warmth map of RNA-sequencing information for genes considerably altered in dTTP synthesis and glutathione metabolism, as decided by Ingenuity Pathway Evaluation (IPA), sorted primarily based on log2 fold modifications >1 or < -1 with adjusted p < 0.05. b A schematic diagram illustrates genes linked to dTTP synthesis and GSH metabolism. Genes upregulated in T315I-Bcr-Abl-32D cells are proven in orange and downregulated in inexperienced. c The RT-qPCR evaluation of genes indicated in (a). Information had been normalized utilizing GAPDH, and the expression ranges had been expressed relative to these in untransformed 32D cells. d Immunoblotting of ATF4 in untransformed, WT-, and T315I-Bcr-Abl reworked 32D cells. T315I-Bcr-Abl-32D cells contaminated with shLacZ and shATF4 lentivirus for 48 h had been subjected to (e) immunoblotting and (f) RT-qPCR evaluation to evaluate PSAT1 and SHMT2 ranges. g RT-qPCR evaluation of SHMT2 and the quantitation of dTTP degree in T315I-Bcr-Abl-32D cells after an infection with shLacZ and shSHMT2 lentivirus. h, i T315I-Bcr-Abl-32D cells after an infection with shLacZ and shATF4 lentivirus for 48 h had been subjected to (h) GSH degree and (i) the DNA fiber evaluation (n = 200, scale bar = 10 μm). The grey triangle signifies the boundary between pink fluorescence and inexperienced fluorescence. Information are introduced as means ± S.D, from three unbiased experiments. Asterisks denote **p < 0.01, ***p < 0.001, or ****p < 0.0001 from unpaired two-tailed Pupil’s t-test.
The ATF4-mediated upregulation of amino acid transporters and one-carbon metabolism, together with SLC1A1, SLC7A8, and SHMT2, presumably will increase the availability of glutamate, cysteine, and glycine, which could act in live performance for the biosynthesis of GSH. We then examined the impact of ATF4 knockdown on GSH degree in T315I-Bcr-Abl-32D cells. In settlement, ATF4 knockdown considerably decreased GSH degree in these cells (Fig. 4h). Furthermore, ATF4 knockdown in T315I-Bcr-Abl cells additionally slowed down DNA replication fork development (Fig. 4i). Thus, the rise of ATF4-mediated built-in stress response (ISR) in T315I-Bcr-Abl 32D cells remodels dTTP and GSH biosynthesis, thus avoiding DNA replication stress.
Glutathione however not dTTP degree determines JMF4073 susceptibility
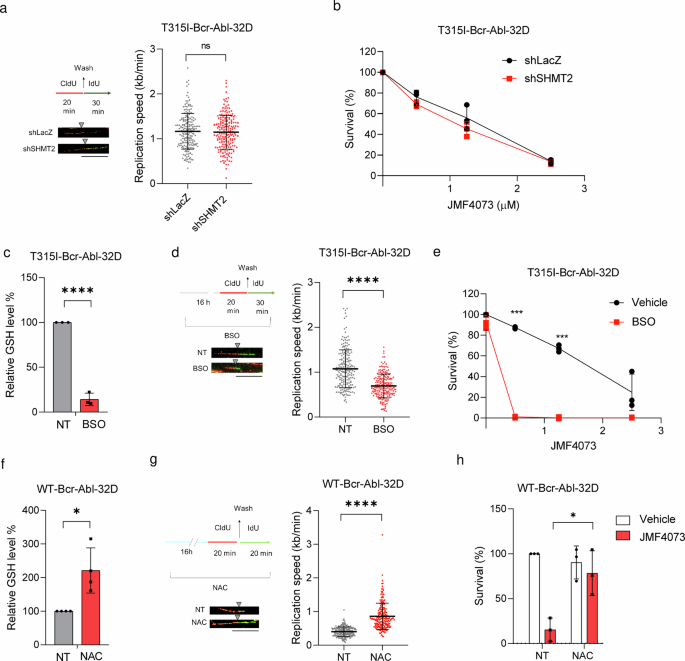
Subsequent, we investigated whether or not the lower in dTTP pool by SHMT2 knockdown may induce DNA replication stress and enhance susceptibility to JMF4073 in T315I-Bcr-Abl-32D cells. Intriguingly, the lower in dTTP swimming pools via SHMT2 knockdown was inadequate to vary the pace of DNA replication fork development and JMF4073 sensitivity in T315I-Bcr-Abl-32D cells (Fig. 5a, b), suggesting that dTTP swimming pools may not be the crucial issue for JMF4073 susceptibility.
T315I-Bcr-Abl-32D cells after an infection with shLacZ and shSHMT2 lentivirus had been examined for (a) DNA fiber evaluation as described within the legend to Fig. 3b (scale bar = 10 μm), and (b) JMF4073 sensitivity evaluation by viability assays. T315I-Bcr-Abl-32D cells after therapy with BSO (12.5 μM) for 16 h had been subjected to (c) the measurement of GSH degree, (d) DNA fiber evaluation, and (e) JMF4073 sensitivity. WT-Bcr-Abl-32D cells after therapy with N-acetyl cysteine (NAC, 1 mM) for 16 h for (f) the measurement of GSH degree, (g) DNA fiber evaluation, and (h) JMF4073 sensitivity. The viability of cells handled with NAC together with JMF4073 (0.5 μM) for 72 h was decided. Information are introduced as means ± S.D., n = 3 organic replicates. Asterisks denote *p < 0.05, **p < 0.01, or ****p < 0.0001 from unpaired two-tailed Pupil’s t-test.
We then examined whether or not the excessive GSH degree is answerable for much less susceptibility to JMF4073 in T315I-Bcr-Abl-32D cells. To this finish, buthionine sulphoximine (BSO), which inhibits γ-glutamylcysteine synthetase36, was used to deal with T315I-Bcr-Abl-32D cells. As anticipated, BSO therapy diminished the mobile degree of GSH along with the lengths of replication tracks (Fig. 5c, d). Furthermore, BSO co-treatment markedly elevated JMF4073 sensitivity (Fig. 5e). Since BSO therapy alone didn’t suppress the expansion of T315I-Bcr-Abl cells, a synergistic impact of JMF4073 together with BSO on elimination of those cells was very clear. Conversely, WT-Bcr-Abl cells after therapy with N-Acetyl-Cysteine (NAC), a GSH precursor that elevated the mobile GSH degree, exhibited not solely elevated lengths of replication tracks but additionally insensitive to JMF4073 (Fig. 5f–h). Altogether, these information recommend that the upregulation of GSH, quite than the dTTP pool, is answerable for avoiding oncogene-induced replication stress and fewer susceptibility to JMF4073 in T315I-Bcr-Abl-32D cells.
Blocking GSH synthesis together with JMF4073 depletes dTTP/dCTP swimming pools to eradicate T315I-Bcr-Abl leukemia
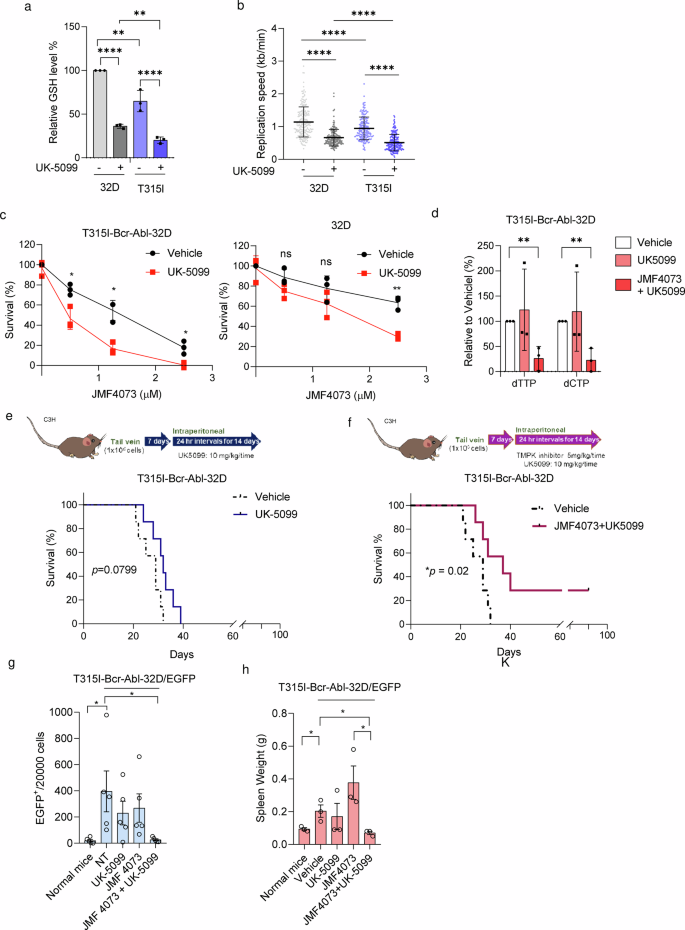
We hypothesize that decreasing the GSH degree to extend replication stress can render T315I-Bcr-Abl leukemia extra vulnerable to JMF4073 in vivo. As talked about, BSO therapy was capable of synergize the impact of JMF4073 on eliminating T315I-Bcr-Abl cells. Nonetheless, we discovered that BSO together with JMF4073 additionally severely suppressed the expansion of 32D myeloid progenitor cells (Supplementary Fig. 4), suggesting the shortage of selectivity for leukemia cells. It has been reported that therapy with UK-5099, an inhibitor of mitochondrial pyruvate service (MPC), reduces the mobile GSH degree by diverting glutamine metabolism from GSH synthesis in tumor cells37. In settlement, UK-5099 therapy was capable of scale back GSH ranges and sluggish replication fork development in T315I-Bcr-Abl cells. It was famous that the results of UK-5099 therapy on GSH discount and replication stress had been much less in 32D cells (Fig. 6a, b). As a consequence, UK-5099 therapy sensitized T315I-Bcr-Abl-32D to JMF4073 however not 32D progenitor cells (Fig. 6c). We additional analyzed dTTP and dCTP pool after the combinatory therapy of UK-5099 with JMF4073 and located the exhaustion of dTTP and dCTP swimming pools in T315I-Bcr-Abl-32D cells (Fig. 6d). We then carried out in vivo remedy of UK-5099 with JMF4073. After transplanting T315I-Bcr-Abl-32D/EGFP cells into C3H/HeNCrNarl mice for 7 days, the mice had been handled with day by day intraperitoneal injections of UK-5099 alone or together with JMF4073 for 14 days (Fig. 6e, f). The survival of T315I leukemia mice was not affected by UK-5099 remedy. As a distinction, the mixture of UK-5099 with JMF4073 remedy extended the survival. Furthermore, mice blood evaluation revealed marked decreases within the inhabitants of T315I-Bcr-Abl-32D/EGFP + cells by the mixture of JMF4073 with UK-5099 therapy (Fig. 6g), together with the spleen measurement (Fig. 6h).
T315I-Bcr-Abl-transformed and untransformed 32D cells had been handled with UK-5099 (25 μM) for 16 h. These cells had been subjected to (a) GSH measurement, and (b) DNA fiber assay. c JMF4073 sensitivity assay. d dTTP and dCTP swimming pools measurement of T315I-Bcr-Abl-32D cells after therapy with car, UK-5099 alone, and the mixture of UK-5099 and JMF4073. e, f C3H/HeNCrNarl mice had been intravenously injected with 1 × 106 cells of T315I-Bcr-Abl-32D cell. After 7 days of transplantation, mice had been handled car (n = 7), UK-5099 (10 mg/kg/time, n = 7) (e) or JMF4073 (5 mg/kg/time) + UK-5099 (10 mg/kg/time) (n = 7) (f) by intraperitoneal injection at 24 h interval for 14 days. The Kaplan–Meier plot reveals the survival of mice with therapy as indicated. g stream cytometry evaluation of peripheral blood (n = 5) and (h) Spleen weights (n = 3) from T315I-Bcr-Abl-32D/EGFP+ bearing mice handled with car, UK-5099, JMF4073, or JMF4073 mixed with UK-5099. The variety of EGFP+ cells in mice with T315I-Bcr-Abl-induced CML was decided on day 30 after transplantation. Information are represented as means ± S.D., n > 3 organic replicates. Asterisks denote *p < 0.05 from unpaired two-tailed Pupil’s t-test or Log-rank (Mantel–Cox) (f, g) check. The mice photos are hand-drawn utilizing the free Samsung PENUP software program.
Artificial lethality by focusing on glutathione and pyrimidylate kinases in human CML-BC cell strains
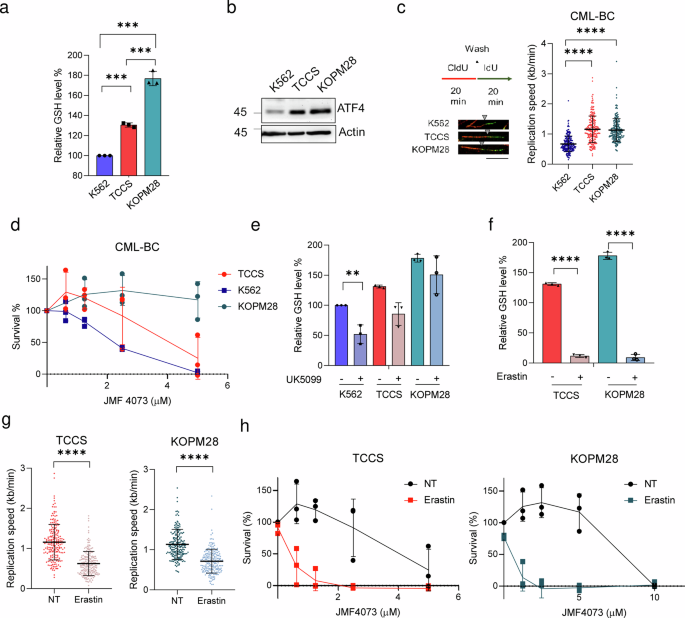
Lastly, we additional used a number of human blast-crisis CML cell strains, together with K562, KOPM28, and TCCS, to confirm the connection between ATF4, GSH, and replication stress. The outcomes confirmed the correlation of ATF4 with GSH degree and the replication fork size. Amongst these human cell strains, the degrees of ATF4 and GSH had been lowest in K562 cells, whereas a lot larger in TCCS and KOPM28 cells (Fig. 7a–c). In settlement, JMF4073 sensitivity was larger in K562, much less in TCCS, and insensitive in KOPM28 (Fig. 7d). Thus, the JMF4073 sensitivity is inversely correlated with ATF4 and GSH ranges. For K562 cells, UK-5099 therapy was enough to convey down the mobile degree of GSH. As for KOPM28 and TCCS cells, GSH degree remained unchanged after UK-5099 therapy (Fig. 7e). We then handled these cells with a low dosage of erastin, the inhibitor of cysteine transporter xCT38, which drastically diminished GSH degree and induced replication stress (Fig.7f, g). We then examined whether or not the therapy with erastin at 0.5 μΜ, a sub-lethal dosage, may sensitize KOPM28 and TCCS CML-BC strains to JMF4073. The outcomes confirmed that erastin markedly elevated the JMF4073 sensitivity in these two CML-BC cell strains (Fig. 7h). Thus, artificial lethality is achieved by co-targeting CMPK/TMPK and GSH in CML-BC cells which have excessive degree of GSH. Alternatively, these information additionally indicate that JMF4073 co-treatment enlarges the therapeutic window of erastin.
K562, TCCS, and KOPM28 had been subjected to (a) GSH measurement, (b) immunoblotting of ATF4, and (c) DNA fiber evaluation. d K562, TCCS and KOPM28 cells had been handled with JMF4073. After 3 days, cells had been analyzed by viability assays. GSH degree in K562, TCCS, and KOPM28 cells after handled with (e) UK-5099 (50 μM) or (f) erastin (0.5 μM) for 16 h for GSH measurement. g After therapy with erastin (0.5 μM) for 16 h, DNA fiber analyses had been carried out in TCCS (n = 200) and KOPM28 cells (n = 200). h JMF4073 sensitivity in TCCS and KOPM28 cells after pretreatment with erastin (0.5 μM) for 16 h. All information are introduced as means ± S.D. from 3 unbiased experiments. Asterisks denote **p < 0.01, or ****p < 0.0001 from unpaired two-tailed Pupil’s t check.