1 Introduction
Non-small cell lung most cancers (NSCLC) accounts for 80%–85% of lung most cancers and is the main explanation for cancer-related dying worldwide (Alexander et al., 2020). Mutations within the epidermal progress issue receptor (EGFR) gene are important targets in NSCLC, affecting practically 50% of East-Asian carriers. Three generations of EGFR tyrosine kinase inhibitors (TKIs) have been developed to focus on completely different delicate mutant websites. The primary-generation reversible EGFR-TKIs, resembling gefitinib, erlotinib, and icotinib, are the first decisions of first-line remedy, providing important survival advantages and good drug security (Gazdar, 2009; Huang et al., 2020). As a result of their excessive incidence of hostile results, the medical software of second-generation EGFR-TKIs, together with afatinib and dacomitinib, is comparatively restricted (Huang et al., 2020). Osimertinib, a third-generation EGFR-TKI, reveals favorable security and wonderful central nervous system (CNS) penetration and is really useful as the brand new first-line remedy of superior NSCLC sufferers in routine observe (Leonetti et al., 2019; Huang et al., 2020).
Nonetheless, improvement of resistance is an inevitable problem in TKI remedy. The acquired resistance mechanisms to osimertinib could be categorized into two main sorts: EGFR-dependent mechanisms, resembling EGFR T790M mutation, and EGFR-independent mechanisms, predominately characterised by MET/HER2 amplification and different bypass activated mutations (Leonetti et al., 2019). In a comparatively small proportion of osimertinib-resistant sufferers, acquired EML4-ALK fusion has been detected (Ho et al., 2017; Minari et al., 2018; Offin et al., 2018). This resistance mechanism has additionally been recognized in sufferers handled with first- and second-generation TKIs. In an early-stage NSCLC affected person with remedy historical past of adjuvant chemotherapy plus gefitinib, twin mutations of ALK-R3HDM1 (A19: R21) and EML4-ALK (E6: A20, variant 3) rearrangement had been detected in his metastatic lymph node. A case that obtained second-line erlotinib and chemotherapy developed acquired EML4-ALK fusion and EGFR 19del mutations (Zeng et al., 2022). EML4-ALK fusion has additionally been reported to function the resistant mechanism to afatinib in an 80-year-old male (Xu et al., 2019). A assessment enrolling 24 NSCLC circumstances reported that three of seven afatinib-relapsed sufferers harbored constructive EML4-ALK fusion (Schrock et al., 2018). For third technology EGFR-TKIs, along with osimertinib, acquired EML4-ALK fusion was additionally recognized in an almonertinib-relapsed NSCLC (Ren et al., 2022). Whether or not influenced by the intensive software in medical observe or TKI generations, sufferers growing acquired EML4-ALK fusion represent a good portion of osimertinib-replased inhabitants (Passaro et al., 2021; Zeng et al., 2022).
The EML4-ALK rearrangement is one other oncogenic driver mutation that happens in 3%–5% of NSCLC and could be focused by ALK-TKIs (McCusker et al., 2019). Crizotinib, a first-generation ALK-TKI, reveals superiority over chemotherapy for ALK-rearranged NSCLC however has restricted results in sufferers with CNS involvement. This limitation has been considerably improved by the second-generation ALK-TKIs, together with ceritinib, alectinib, brigatinib, and ensartinib. Moreover, lorlatinib, a third-generation ALK-TKI, has been developed to beat the first and bought resistance to earlier-generation ALK inhibitors (Wu et al., 2016). Previous to the demonstration of the protection and superior efficacy of second- and third-generation ALK-TKIs in medical trials, crizotinib was the first first-line therapeutic technique for treatment-naive sufferers. Consequently, in beforehand reported circumstances of osimertinib-resistant sufferers growing novel EML4-ALK fusion, crizotinib was administered orally. Regrettably, these circumstances didn’t obtain sturdy drug responses. As an illustration, following osimertinib failure and the detection of EML4-ALK variant 1 (V1), crizotinib was administrated as a posterior line remedy for a smoker affected person, leading to solely a 4-month profit, adopted by brigatinib (Yan et al., 2020). Likewise, a transient response of crizotinib plus osimertinib was noticed in a progressed lung adenocarcinoma affected person beforehand handled with first-line gefitinib and second-line osimertinib (Hou et al., 2021).
Just lately, the first-line remedy choices for major tumors with ALK rearrangement have expanded to incorporate second- and third-generation ALK-TKIs (Peters et al., 2017; Camidge et al., 2018; Horn et al., 2021; Solomon et al., 2023). Consequently, the therapeutic strategy for osimertinib-resistant sufferers with acquired EML4-ALK fusion is not restricted to crizotinib. Amongst these options, alectinib has been administrated to a number of sufferers; nevertheless, it exhibited medical actions for lower than 5 months (von Buttlar et al., 2021; Wang et al., 2023). Regardless of the low frequency of acquired EML4-ALK fusion in osimertinib-resistant circumstances, the numerous variety of sufferers with EGFR mutations bear osimertinib remedy, together with the unfavorable medical outcomes related to subsequent crizotinib or alectinib remedy, underscores the need for additional exploration of simpler remedy methods to enhance medical outcomes.
Right here, we report a case of resectable NSCLC harboring advanced EGFR L858R/G719S, and BRAF V600E mutations. Throughout adjuvant remedy, the affected person skilled long-term advantages from the sequential monotherapy with erlotinib, osimertinib, and ensartinib. Notably, the third-line ensartinib demonstrated considerably improved efficacy and security on this affected person, who developed EML4-ALK fusion following resistance to osimertinib, offering precious steering for medical choices in sufferers administration.
2 Case presentation
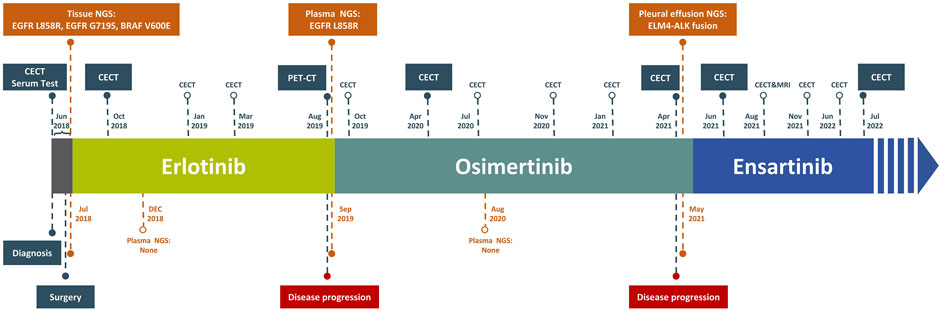
On 5 June 2018, a 71-year-old Chinese language feminine with no smoking historical past was admitted to our hospital presenting power cough and chest misery. Her medical historical past included hepatitis B, which had been in remission for 30 years, and a secure benign meningioma, with no remedy historical past. One month earlier than admission, a fluorine 18 (18F)-labeled fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) recognized a major space-occupying lesion with irregular margins (1.7*1.8 cm) in her left decrease lobe close to the pleura. Moreover, three lymph nodes in mediastinum and left hilum confirmed indicators of metastasis, with no distant metastases noticed. Based mostly on PET-CT scans, this affected person was identified with stage IIIA peripheral lung most cancers with lymph node metastasis. A contrast-enhanced CT (CECT) scan additional confirmed the presence of a nodule within the left decrease lobe (Determine 1B). Small nodules in the proper higher lung and adrenal glands weren’t detected by PET-CT, requiring additional remark. Serum biochemistry exams revealed elevated carcinoembryonic antigen (CEA) and cytokeratin fragment 21–1 (Cyfra21-1), exceeding regular ranges.
Determine 1. Sequential CT scan analysis and histological examination evaluation on this case report. (A) Pelvic puncture tissue biopsy. The CT scan picture on June 2018 (B), October 2018 (C), April 2020 (E), April 2021 (F), June 2021 (G), and July 2022 (H). (D) The PET-CT scan on August.
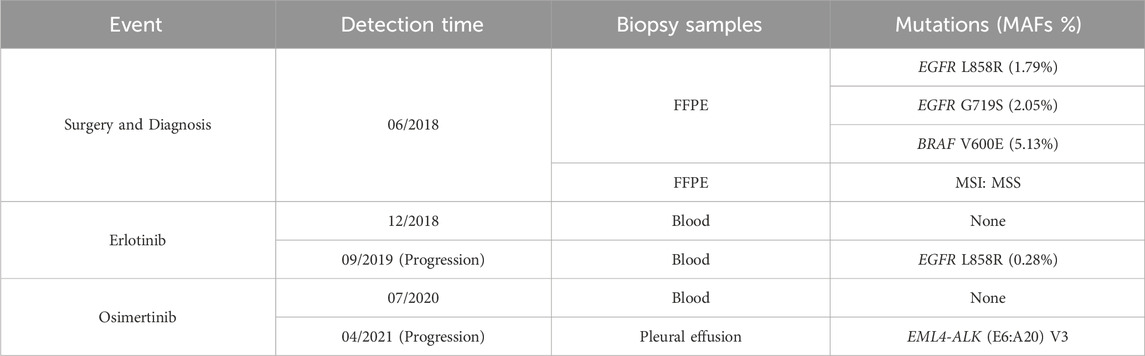
On 11 June 2018, with knowledgeable consent, this affected person underwent a left lung lobotomy and full mediastinal lymphadenectomy (Determine 2). Hematoxylin and eosin staining of the excised tumor mass confirmed a stage IIIA infiltrating lung adenocarcinoma of the stable predominant subtype (Determine 1A), with carcinoma metastasis in regional lymph nodes. Panel-based genomic DNA sequencing of tissue samples revealed compound somatic delicate mutations (Repugene Expertise, Hangzhou, Folks’s Republic of China), together with EGFR L858R/G719S and BRAF V600E (Desk 1). Given the excessive recurrence threat, she obtained adjuvant remedy with erlotinib. 4 months after initiating focused remedy, imaging confirmed no tumor lesions in situ, confirmed by the detrimental outcomes of non-invasive liquid biopsy sequencing performed 2 months later (Determine 1C; Desk 1).
After 13-month remedy of erlotinib, the PET-CT indicated intense FDG exercise in nodules situated within the inferior lobe of the left lung close to the hilar, suggesting intrapulmonary lymph node metastases (Determine 1D). A subsequent plasma genotyping detected a recurring L858R mutation (Desk 1). Osimertinib was tentatively employed as second-line remedy in September 2019, with important tumor discount noticed 7 months later (Determine 1E). Ten months after initiating osimertinib, magnetic resonance imaging of the thoracolumbar vertebrae and routine CT scans revealed slight enlargement of pulmonary nodules, mildly elevated bilateral pleural effusions, and potential bone metastases. Along with continued osimertinib, pleural bevacizumab injections had been began in August 2020 (10-month after initiating osimertinib) to manage pleural effusions, administered month-to-month. After 19 months of osimertinib remedy, the affected person developed new nodules and exhibited clearly elevated bilateral pleural effusions, suggesting potential illness recurrence (Determine 1F). To find out the genetic alternations and subsequent therapeutic methods, pleural effusion was sampled, and the supernatant was subjected to focused panel sequencing in April 2021, which detected an acquired EML4-ALK variant 3 (E6:A20) mutation. In consequence, ensartinib was administered in Might 2021. One month later, a CECT evaluation confirmed alleviated tumor lesion, decreased pleural effusions, and lowered nodules sizes (Determine 1G). Six months after initiating ensartinib, CT scan indicated additional lowered pleural effusions and cleared nodules. At subsequent follow-up visits at 9- and 12-month post-ensartinib remedy, pleural effusions had been persistently decreased. On the final follow-up in July 2022, CT scans confirmed resolved pleural effusions (Determine 1H), and serum marker ranges remained inside regular vary. Along with tolerable rash and minor abnormalities in liver and kidney perform, no different unwanted side effects had been noticed over ensartinib remedy, indicating a positive drug security.
3 Dialogue
Focused remedy characterised by decrease toxicity and bodily burden is a regular remedy routine for NSCLC sufferers carrying driver mutations with medical significance. Nonetheless, the inevitable improvement of resistance necessitates shut monitoring by way of imaging and genetic testing. The event of sequential therapies for long-term remedy advantages from ongoing drug analysis and developments in genetic testing applied sciences, particularly non-invasive liquid biopsy. On this examine, genotyping of resected tumor tissue from an aged feminine affected person identified with lung adenocarcinoma reported the co-mutations of EGFR L858R/G719S and BRAF V600E. Dynamic monitoring through liquid biopsies recognized L858R and EML4-ALK fusion as resistance mechanisms to erlotinib and osimertinib, respectively. The EML4-ALK fusion, an rare off-target alteration to osimertinib, was successfully focused by ensartinib, attaining a 14-month disease-free survival, superior to crizotinib and aletinib in related circumstances reported beforehand. This report of ensartinib’s medical software in osimertinib-resistant affected person gives precious insights for decision-making in medical administration.
The detection of coexisting mutations of EGFR L858R/G719S and BRAF V600E on this affected person posed a problem in selecting the suitable TKI for adjuvant remedy. BRAF, a key molecule within the EGFR/RAS downstream signaling pathway, is mutated in roughly 1.5%–5.5% NSCLC circumstances, with the predominant genotype being BRAF V600E (Jordan et al., 2017; Lin et al., 2019). Promising efficacy and security have been demonstrated with BRAF inhibitors, resembling dabrafenib (Planchard Okay. et al., 2016), vemurafenib (Subbiah et al., 2019), and the mix of dabrafenib plus trametinib (Planchard B. et al., 2016; Planchard et al., 2017; Ettinger et al., 2021), in medical trials involving superior BRAF V600E mutant NSCLC. The event of acquired BRAF V600E has been reported to render tumor insensitivity to EGFR-TKIs remedy (Ohashi et al., 2012; Ricordel et al., 2018; Westover et al., 2018), indicating potential medical advantages of EGFR and BARF co-inhibition in circumstances with twin mutations. Certainly, an growing variety of circumstances have reported that superior EGFR-mutant NSCLC sufferers that developed concomitant BRAF mutations following acquired resistance to EGFR-TKI remedy exhibit sturdy responses and good tolerance to mixed EGFR and BRAF inhibitors remedy (Aboubakar Nana and Ocak, 2021; Ribeiro et al., 2021; Schaufler et al., 2021). As an illustration, a male affected person with lung adenocarcinoma underwent genetic testing after osimertinib remedy failure, which revealed co-mutations of EGFR T790M/19del and BRAF V600E. Consequently, he switched to a mix remedy of debrafenib, trametinib, and osimertinib, that resulted in an entire remission (Meng et al., 2020). One other feminine affected person who developed resistance to osimertinib was discovered to hold EGFR 19del plus BRAF V600E mutations, obtained debrafenib, trametinib, and osimertinib, and achieved a secure illness inside 6-month of remedy (Zeng et al., 2021). These findings point out that, in our case, introducing BRAF inhibitors may assist enhance survival advantages when mixed with erlotinib. Nonetheless, there’s a lack of complete medical trials on this mixture remedy, and the obtainable medical knowledge are restricted, significantly in circumstances with major BRAF and EGFR mutations. Provided that the resistance mechanisms of debrafenib, trametinib, and EGFR-TKI remained unclear which can restrict subsequent therapeutic choices, this affected person opted for erlotinib monotherapy. A medical response of 14 months confirmed that erlotinib is an efficient remedy.
On the finish of erlotinib remedy, plasma genotyping was performed to information the change in therapeutic technique because of the lack of tumor tissues, and this testing confirmed the presence of EGFR L858R mutation. Based on a diagnostic evaluation of 216 superior NSCLC circumstances, liquid biopsies have proven a 96.5% specificity in detecting L858R (Schrock et al., 2018), suggesting the reliability of this consequence for our affected person. Osimertinib has demonstrated superior efficacy over first- and second-generation EGFR-TKIs in medical trials of superior NSCLC with L858R mutations. The FLAURA examine, which targeted on treatment-naive sufferers with L858R, discovered that these handled with osimertinib had a protracted PFS than these receiving normal EGFR-TKI (gefitinib or erlotinib) (14.4 months vs. 9.5 months) (Soria et al., 2018). One other meta-analysis additionally reported the longest PFS in osimertinib-treated group amongst 12 remedy methods (Zhao Y. et al., 2019). These findings recommend that osimertinib may present a sturdy and protected response for this affected person and we began osimertinib remedy with affected person’s consent. In the course of the course of remedy, regardless of minor radiographic indicators of development, we continued osimertinib as no driver mutations had been detectable within the plasma genetic testing. Nonetheless, 19 months after initiating osimertinib, an EML4-ALK fusion emerged, indicating a change within the tumor’s genetic panorama.
The resistance mechanisms of second-line osimertinib have been systematically documented, reporting that numerous oncogene fusion occasions, together with ALK fusions, happen in lower than 10% of circumstances (Fu et al., 2022). In situations the place sufferers developed acquired EML4-ALK fusion after osimertinib, these sometimes co-occurred with EGFR mutations resembling T790M, L858R, and 19del. Consequently, a mix of osimertinib plus ALK inhibitors is employed. Nonetheless, most sufferers skilled tumor development inside 6 months when osimertinib is mixed with crizotinib or alectinib (Batra et al., 2020; Hou et al., 2021; von Buttlar et al., 2021; Wang et al., 2023; Yan et al., 2020). For instance, one affected person confirmed illness development on CT scans simply 1 month after beginning second-line remedy with osimertinib and crizotinib, and switching to brigatinib solely maintained a secure illness response for 4 months (Yan et al., 2020). Ensartinib, a newly-marketed second-generation ALK inhibitor, has proven superior efficacy to crizotinib in first-line remedy for each systemic and intracranial illness. It has additionally confirmed efficient in circumstances immune to crizotinib or in overcoming alectinib-induced hostile occasions (Batra et al., 2020; Yang et al., 2020; Horn et al., 2021). In mild of this, our affected person obtained ensartinib and maintained high quality of life over a 14-month follow-up, displaying higher outcomes in comparison with prior circumstances handled with crizotinib or brigatinib after osimertinib resistance. It has been reported that one other NSCLC affected person, who developed EML4-ALK fusion after turning into resistant to a different third-generation EGFR-TKI almonertinib, obtained crizotinib mixed with almonertinib however solely had a secure illness standing for 1 month (Ren et al., 2022). It is a far much less efficient case, although you will need to acknowledge that the various effectiveness between ensartinib and different ALK inhibitors is likely to be influenced by the presence of concurrent EGFR mutations, in addition to patient-specific elements. The efficacy of ensartinib in osimertinib-resistant sufferers with co-existing EGFR and ALK mutations requires additional exploration.
In the course of the medical analysis and administration of this case, a number of limitations should be addressed. First, the preoperative medical analysis of this affected person was solely based mostly on CT scans with out histopathological affirmation by way of tissue biopsy, which may have offered a extra definitive analysis. Second, the clinically important mutations recognized by next-generation sequencing weren’t validated utilizing different strategies resembling Sanger sequencing and quantitative polymerase chain response, which may have strengthened the reliability of the outcomes. Lastly, the genotyping performed utilizing blood or pleural effusion samples weren’t additional confirmed in tumor tissues, elevating the potential for false-negative outcomes.
4 Conclusion
This case report particulars a NSCLC affected person with advanced driver mutations, particularly, EGFR L858R/G719S and BRAF V600E, within the major tumor. Remedy concerned sequential monotherapies of erlotinib, osimertinib, and ensartinib, guided by non-invasive liquid biopsies. Of word, the emergence of an EML4-ALK rearrangement as a resistance mechanism to osimertinib remedy was successfully focused by ensartinib, offering a focused answer to this particular resistance mechanism. This technique underscores the potential of tailor-made therapies based mostly on evolving genetic profiles in managing advanced NSCLC circumstances.
Information availability assertion
The unique contributions offered within the examine are included within the article; additional inquiries could be directed to the corresponding creator.
Ethics assertion
The research involving people had been accepted by the Tianjin Medical College Most cancers Institute and Hospital. The research had been performed in accordance with the native laws and institutional necessities. The individuals offered their written knowledgeable consent to take part on this examine. Written knowledgeable consent was obtained from the person(s) for the publication of any probably identifiable photographs or knowledge included on this article.
Writer contributions
YG: Information curation, Investigation, Writing–unique draft. RZ: Information curation, Investigation, Writing–unique draft. YM: Writing–assessment and modifying, Conceptualization, Supervision. LW: Writing–assessment and modifying. LZ: Writing–assessment and modifying. JY: Conceptualization, Supervision, Writing–assessment and modifying.
Funding
The creator(s) declare monetary help was obtained for the analysis, authorship, and/or publication of this text. This examine was supported by Wu Jieping Medical Basis (Grant quantity: 320.6750.2022-09-26).
Battle of curiosity
Authors YM, LW, and LZ had been employed by Hangzhou Repugene Expertise Co., Ltd.
The remaining authors declare that the analysis was performed within the absence of any business or monetary relationships that might be construed as a possible battle of curiosity.
Writer’s word
All claims expressed on this article are solely these of the authors and don’t essentially characterize these of their affiliated organizations, or these of the writer, the editors and the reviewers. Any product that could be evaluated on this article, or declare that could be made by its producer, will not be assured or endorsed by the writer.
References
Aboubakar Nana, F., and Ocak, S. (2021). Focusing on BRAF activation as acquired resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small-cell lung most cancers. Pharmaceutics 13 (9), 1478. doi:10.3390/pharmaceutics13091478
PubMed Summary | CrossRef Full Textual content | Google Scholar
Batra, U., Sharma, M., Amrith, B. P., Mehta, A., and Jain, P. (2020). EML4-ALK fusion as a resistance mechanism to osimertinib and its profitable administration with osimertinib and alectinib: case report and assessment of the literature. Clin. Lung Most cancers 21 (6), e597–e600. doi:10.1016/j.cllc.2020.05.016
PubMed Summary | CrossRef Full Textual content | Google Scholar
Camidge, D. R., Kim, H. R., Ahn, M. J., Yang, J. C., Han, J. Y., Lee, J. S., et al. (2018). Brigatinib versus crizotinib in ALK-positive non-small-cell lung most cancers. N. Engl. J. Med. 379 (21), 2027–2039. doi:10.1056/NEJMoa1810171
PubMed Summary | CrossRef Full Textual content | Google Scholar
Ettinger, D. S., Wooden, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., et al. (2021). NCCN pointers insights: non-small cell lung most cancers, model 2.2021. J. Natl. Compr. Canc Netw. 19 (3), 254–266. doi:10.6004/jnccn.2021.0013
PubMed Summary | CrossRef Full Textual content | Google Scholar
Fu, Okay., Xie, F., Wang, F., and Fu, L. (2022). Therapeutic methods for EGFR-mutated non-small cell lung most cancers sufferers with osimertinib resistance. J. Hematol. Oncol. 15 (1), 173. doi:10.1186/s13045-022-01391-4
PubMed Summary | CrossRef Full Textual content | Google Scholar
Gazdar, A. F. (2009). Activating and resistance mutations of EGFR in non-small-cell lung most cancers: function in medical response to EGFR tyrosine kinase inhibitors. Oncogene 28 (Suppl 1), S24–S31. doi:10.1038/onc.2009.198
PubMed Summary | CrossRef Full Textual content | Google Scholar
Ho, C. C., Liao, W. Y., Lin, C. A., Shih, J. Y., Yu, C. J., and Yang, J. C. (2017). Acquired BRAF V600E mutation as resistant mechanism after remedy with osimertinib. J. Thorac. Oncol. 12 (3), 567–572. doi:10.1016/j.jtho.2016.11.2231
PubMed Summary | CrossRef Full Textual content | Google Scholar
Horn, L., Wang, Z., Wu, G., Poddubskaya, E., Mok, T., Reck, M., et al. (2021a). Ensartinib vs crizotinib for sufferers with anaplastic lymphoma kinase-positive non-small cell lung most cancers: a randomized medical trial. JAMA Oncol. 7 (11), 1617–1625. doi:10.1001/jamaoncol.2021.3523
PubMed Summary | CrossRef Full Textual content | Google Scholar
Hou, H., Solar, D., Zhang, C., Liu, D., and Zhang, X. (2021). ALK rearrangements as mechanisms of acquired resistance to osimertinib in EGFR mutant non-small cell lung most cancers. Thorac. Most cancers 12 (6), 962–969. doi:10.1111/1759-7714.13817
PubMed Summary | CrossRef Full Textual content | Google Scholar
Huang, L., Jiang, S., and Shi, Y. (2020). Tyrosine kinase inhibitors for stable tumors up to now 20 years (2001-2020). J. Hematol. Oncol. 13 (1), 143. doi:10.1186/s13045-020-00977-0
PubMed Summary | CrossRef Full Textual content | Google Scholar
Jordan, E. J., Kim, H. R., Arcila, M. E., Barron, D., Chakravarty, D., Gao, J., et al. (2017). Potential complete molecular characterization of lung adenocarcinomas for environment friendly affected person matching to accepted and rising therapies. Most cancers Discov. 7 (6), 596–609. doi:10.1158/2159-8290.CD-16-1337
PubMed Summary | CrossRef Full Textual content | Google Scholar
Leonetti, A., Sharma, S., Minari, R., Perego, P., Giovannetti, E., and Tiseo, M. (2019). Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung most cancers. Br. J. Most cancers 121 (9), 725–737. doi:10.1038/s41416-019-0573-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
Lin, Q., Zhang, H., Ding, H., Qian, J., Lizaso, A., Lin, J., et al. (2019). The affiliation between BRAF mutation class and medical options in BRAF-mutant Chinese language non-small cell lung most cancers sufferers. J. Transl. Med. 17 (1), 298. doi:10.1186/s12967-019-2036-7
PubMed Summary | CrossRef Full Textual content | Google Scholar
McCusker, M. G., Russo, A., Scilla, Okay. A., Mehra, R., and Rolfo, C. (2019). How I deal with ALK-positive non-small cell lung most cancers. ESMO Open 4 (Suppl 2), e000524. doi:10.1136/esmoopen-2019-000524
PubMed Summary | CrossRef Full Textual content | Google Scholar
Meng, P., Koopman, B., Kok, Okay., Ter Elst, A., Schuuring, E., van Kempen, L. C., et al. (2020). Mixed osimertinib, dabrafenib and trametinib remedy for superior non-small-cell lung most cancers sufferers with an osimertinib-induced BRAF V600E mutation. Lung Most cancers 146, 358–361. doi:10.1016/j.lungcan.2020.05.036
PubMed Summary | CrossRef Full Textual content | Google Scholar
Minari, R., Bordi, P., La Monica, S., Squadrilli, A., Leonetti, A., Bottarelli, L., et al. (2018). Concurrent acquired BRAF V600E mutation and MET amplification as resistance mechanism of first-line osimertinib remedy in a affected person with EGFR-mutated NSCLC. J. Thorac. Oncol. 13 (6), e89–e91. doi:10.1016/j.jtho.2018.03.013
PubMed Summary | CrossRef Full Textual content | Google Scholar
Offin, M., Somwar, R., Rekhtman, N., Benayed, R., Chang, J. C., Plodkowski, A., et al. (2018). Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR-mutant lung cancers. JCO Summary. Oncol. 2, 1–12. doi:10.1200/PO.18.00126
Ohashi, Okay., Sequist, L. V., Arcila, M. E., Moran, T., Chmielecki, J., Lin, Y. L., et al. (2012). Lung cancers with acquired resistance to EGFR inhibitors often harbor BRAF gene mutations however lack mutations in KRAS, NRAS, or MEK1. Proc. Natl. Acad. Sci. U S A 109 (31), E2127–E2133. doi:10.1073/pnas.1203530109
PubMed Summary | CrossRef Full Textual content | Google Scholar
Peters, S., Camidge, D. R., Shaw, A. T., Gadgeel, S., Ahn, J. S., Kim, D. W., et al. (2017). Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung most cancers. N. Engl. J. Med. 377 (9), 829–838. doi:10.1056/NEJMoa1704795
PubMed Summary | CrossRef Full Textual content | Google Scholar
Planchard, D., Besse, B., Groen, H. J. M., Souquet, P. J., Quoix, E., Baik, C. S., et al. (2016). Dabrafenib plus trametinib in sufferers with beforehand handled BRAF(V600E)-mutant metastatic non-small cell lung most cancers: an open-label, multicentre part 2 trial. Lancet Oncol. 17 (7), 984–993. doi:10.1016/S1470-2045(16)30146-2
PubMed Summary | CrossRef Full Textual content | Google Scholar
Planchard, D., Kim, T. M., Mazieres, J., Quoix, E., Riely, G., Barlesi, F., et al. (2016). Dabrafenib in sufferers with BRAF(V600E)-positive superior non-small-cell lung most cancers: a single-arm, multicentre, open-label, part 2 trial. Lancet Oncol. 17 (5), 642–650. doi:10.1016/S1470-2045(16)00077-2
PubMed Summary | CrossRef Full Textual content | Google Scholar
Planchard, D., Smit, E. F., Groen, H. J. M., Mazieres, J., Besse, B., Helland, A., et al. (2017). Dabrafenib plus trametinib in sufferers with beforehand untreated BRAF(V600E)-mutant metastatic non-small-cell lung most cancers: an open-label, part 2 trial. Lancet Oncol. 18 (10), 1307–1316. doi:10.1016/S1470-2045(17)30679-4
PubMed Summary | CrossRef Full Textual content | Google Scholar
Ren, Okay. H., Qin, W. W., Wang, Y., Peng, J. C., and Hu, W. X. (2022). Detection of an EML4-ALK fusion mutation secondary to epidermal progress issue receptor-tyrosine kinase inhibitor (EGFR-TKI) remedy for lung most cancers: a case report. Ann. Palliat. Med. 11 (7), 2503–2509. doi:10.21037/apm-22-744
PubMed Summary | CrossRef Full Textual content | Google Scholar
Ribeiro, M., Knebel, F. H., Bettoni, F., Saddi, R., Sacardo, Okay. P., Canedo, F., et al. (2021). Spectacular response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma affected person. NPJ Summary. Oncol. 5 (1), 5. doi:10.1038/s41698-021-00149-4
PubMed Summary | CrossRef Full Textual content | Google Scholar
Ricordel, C., Friboulet, L., Facchinetti, F., and Soria, J. C. (2018). Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung most cancers. Ann. Oncol. 29 (suppl_1), i28–i37. doi:10.1093/annonc/mdx705
PubMed Summary | CrossRef Full Textual content | Google Scholar
Schaufler, D., Ast, D. F., Tumbrink, H. L., Abedpour, N., Maas, L., Schwabe, A. E., et al. (2021). Clonal dynamics of BRAF-driven drug resistance in EGFR-mutant lung most cancers. NPJ Summary. Oncol. 5 (1), 102. doi:10.1038/s41698-021-00241-9
PubMed Summary | CrossRef Full Textual content | Google Scholar
Schrock, A. B., Zhu, V. W., Hsieh, W. S., Madison, R., Creelan, B., Silberberg, J., et al. (2018). Receptor tyrosine kinase fusions and BRAF kinase fusions are uncommon however actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 13 (9), 1312–1323. doi:10.1016/j.jtho.2018.05.027
PubMed Summary | CrossRef Full Textual content | Google Scholar
Solomon, B. J., Bauer, T. M., Mok, T. S. Okay., Liu, G., Mazieres, J., de Marinis, F., et al. (2023). Efficacy and security of first-line lorlatinib versus crizotinib in sufferers with superior, ALK-positive non-small-cell lung most cancers: up to date evaluation of information from the part 3, randomised, open-label CROWN examine. Lancet Respir. Med. 11 (4), 354–366. doi:10.1016/S2213-2600(22)00437-4
PubMed Summary | CrossRef Full Textual content | Google Scholar
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, Okay. H., et al. (2018). Osimertinib in untreated EGFR-mutated superior non-small-cell lung most cancers. N. Engl. J. Med. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
PubMed Summary | CrossRef Full Textual content | Google Scholar
Subbiah, V., Gervais, R., Riely, G., Hollebecque, A., Blay, J. Y., Felip, E., et al. (2019). Efficacy of vemurafenib in sufferers with non-small-cell lung most cancers with BRAF V600 mutation: an open-label, single-arm cohort of the histology-independent VE-BASKET examine. JCO Summary. Oncol. 3, 1–9. doi:10.1200/PO.18.00266
PubMed Summary | CrossRef Full Textual content | Google Scholar
von Buttlar, X., Reuss, J. E., Liu, S. V., and Kim, C. (2021). EML4-ALK rearrangement as a mechanism of resistance to osimertinib in metastatic lung adenocarcinoma: a case report. JTO Clin. Res. Rep. 2 (6), 100179. doi:10.1016/j.jtocrr.2021.100179
PubMed Summary | CrossRef Full Textual content | Google Scholar
Wang, L.-S., Chen, S.-Q., Zhong, X., Jiao, X. D., Liu, Okay., Qin, B. D., et al. (2023). Acquired EML4-ALK fusion and EGFR C797S in cis mutation as resistance mechanisms to osimertinib in a non-small cell lung most cancers affected person with EGFR L858R/T790M. Anticancer Medicine 34 (10), 1146–1150. doi:10.1097/CAD.0000000000001489
PubMed Summary | CrossRef Full Textual content | Google Scholar
Westover, D., Zugazagoitia, J., Cho, B. C., Lovly, C. M., and Paz-Ares, L. (2018). Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 1 (suppl_1), i10–i19. doi:10.1093/annonc/mdx703
PubMed Summary | CrossRef Full Textual content | Google Scholar
Xu, H., Shen, J., Xiang, J., Li, H., Li, B., Zhang, T., et al. (2019). Characterization of acquired receptor tyrosine-kinase fusions as mechanisms of resistance to EGFR tyrosine-kinase inhibitors. Most cancers Manag. Res. 11, 6343–6351. doi:10.2147/CMAR.S197337
PubMed Summary | CrossRef Full Textual content | Google Scholar
Yan, Y., Jiang, G., Ma, W., Li, T., and Wang, L. (2020). Rising EML4-ALK Variant 5 as a Concurrent Resistance Mechanism to Osimertinib in a Affected person With EGFR E19del/T790M NSCLC. Clin. Lung Most cancers 21 (6), 562–567. doi:10.1016/j.cllc.2020.05.009
PubMed Summary | CrossRef Full Textual content | Google Scholar
Yang, Y., Zhou, J., Zhou, J., Feng, J., Zhuang, W., Chen, J., et al. (2020). Efficacy, security, and biomarker evaluation of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung most cancers: a multicentre, part 2 trial. Lancet Respir. Med. 8 (1), 45–53. doi:10.1016/S2213-2600(19)30252-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
Zeng, R., Luo, L., Solar, X., Bao, Z., Du, W., Dai, R., et al. (2021). EGFR/BRAF/MEK co-inhibition for EGFR-mutated lung adenocarcinoma sufferers with an acquired BRAF(V600E) mutation: a case report and assessment of literature. Most cancers Drug Resist 4 (4), 1019–1027. doi:10.20517/cdr.2021.98
PubMed Summary | CrossRef Full Textual content | Google Scholar
Zeng, Z., Wang, T., He, J., and Wang, Y. (2022). ALK-R3HDM1 and EML4-ALK fusion as a mechanism of acquired resistance to gefitinib: a case report and literature assessment. Entrance. Oncol. 12, 1010084. doi:10.3389/fonc.2022.1010084
PubMed Summary | CrossRef Full Textual content | Google Scholar
Zhao Y, L. J., Cai, X., Pan, Z., Liu, J., Yin, W., Chen, H., et al. (2019). Efficacy and security of first line therapies for sufferers with superior epidermal progress issue receptor mutated, non-small cell lung most cancers: systematic assessment and community meta-analysis. BMJ 367, l5460. doi:10.1136/bmj.l5460
PubMed Summary | CrossRef Full Textual content | Google Scholar