Acute myeloid leukemia (AML) is a malignancy with a poor prognosis. The growth of leukemic cells is related to the impairment of regular hematopoiesis, which might result in extreme morbidity in affected sufferers. Latest research instructed that leukemic cells can rework the bone marrow (BM) microenvironment, the so-called BM area of interest, creating permissive situations favoring leukemic stem cell growth over regular hematopoietic stem and progenitor cells (HSPCs) [1]. BM area of interest is a posh three-dimensional (3D) construction that gives components crucial for the upkeep, survival, and differentiation of wholesome HSPCs [2]. Many of the BM area of interest research have been carried out utilizing murine fashions. Mouse BM area of interest is outlined primarily based on the anatomical localization of various cell varieties and contains mesenchymal stromal cells (MSCs), endothelial cells, macrophages, lymphocytes, and megakaryocytes [2]. As well as, sensory and sympathetic nerve fibers (SNF) penetrating the BM have been described for the primary time in animals greater than 50 years in the past [3]. Later, neuropeptide Y-expressing neurons and parasympathetic nerve fibers have been detected in rat femurs [4]. The latest growth of 3D imaging means that the sympathetic nervous system regulates balanced blood cell manufacturing [5], HSPC migration [6], and regeneration of hematopoiesis in response to genotoxic stresses [7] in murine fashions. Sensory signaling has been reported to modulate the performance of MSCs and osteoclasts in murine BM [4]. Within the case of AML, it was just lately proven in a murine mannequin generated by retroviral an infection of HSPCs with MLL-AF9 that purposeful SNFs have been disrupted round arterioles, resulting in the growth of MSCs and endothelial cells [8]. Due to this fact, it’s cheap to hypothesize that SNFs doubtlessly may regulate hematopoiesis in human BM.
Essentially the most studied human BM area of interest part is MSCs, as they play a central position within the management of HSPC destiny by direct interplay and thru the secretion of soluble components [2]. It was proven that MSCs from AML sufferers have a decreased clonogenic capability and proliferate lower than MSCs from wholesome donors in vitro. Nonetheless, it’s nonetheless unknown whether or not MSCs upon malignancy have tumor-promoting or tumor-suppressing results. A number of research instructed that MSCs have the power to type a most cancers stem cell area of interest in xenograft mouse fashions and promote leukemia cell progress. On the similar time, anti-inflammatory cytokines secreted by MSCs might trigger cell cycle arrest of most cancers cells, thus inhibiting tumor progress [1]. Moreover, in mouse fashions of AML and myeloproliferative neoplasm, it has been proven that leukemia clones set off harm of SNFs, which consequently impacts the variety of MSCs and endothelial cells within the BM, resulting in suppression of regular hematopoiesis with a concomitant proliferation of mutant cells [8, 9].
Regardless of detailed characterization of murine BM microenvironment in regular state and tumor fashions, the 3D structure of wholesome and leukemia human BM area of interest stays underexplored as a result of problem of acquiring BM biopsies, as many clinics have shifted from performing biopsies for prognosis to primarily utilizing BM aspirates. Thus, helpful details about the human BM area of interest, which can’t be obtained from a BM aspirate alone, is lacking. This research aimed to research the human BM area of interest 3D structure, together with MSC distribution and sympathetic innervation in AML sufferers’ BM at prognosis, throughout, and after cytotoxic remedy (CT).
To visualise the human BM area of interest structure, we used 2-photon multicolor confocal microscopy, which permits us to penetrate the biopsy samples as much as 300 μm deep with z-stack intervals of two μm. First, we investigated the distribution of MSCs. Till now, the research of human MSCs stay very heterogeneous due to the shortage of distinctive and definitive mobile markers for his or her identification. Whereas some researchers think about CD271 (low-affinity nerve progress issue receptor) as a pan-MSC marker [10], others use CD90 (Thy-1) for clonogenic MSCs [11] and counsel that almost all of them don’t specific CD271 [12]. The discrepancy within the MSC phenotype most likely comes from the totally different procedures for MSC isolation or totally different tradition situations, as most investigations have been executed in vitro. As well as, the exact localization of MSCs expressing CD271 or CD90 in human BM stays unknown. Because of the problem of acquiring BM biopsy samples from wholesome people, we used samples from three sufferers with lymphoma and one with tubular adenoma with out BM infiltration earlier than any remedy as a management BM (n = 4, age: median = 56 years (vary: 38–61), intercourse: male = 3, feminine = 1) (Suppl. Desk 1). We stained trephine biopsy samples with anti-CD90 and CD271 antibodies to analyze MSC distribution. We discovered that CD45- CD271+ cells, which localize alongside the lengthy cylindrical constructions typical for BM vessels, specific CD90, whereas most CD45– CD271+ CD90– cells are distributed irregularly in mobile parenchyma (Fig. 1A, B; Fig. S1 and Suppl. Video 1). Due to this fact, we advise that CD271 can be utilized as a pan-MSC marker, whereas CD90 likely defines perivascular cells.
A, B Authentic consultant z-stack confocal photographs from human BM biopsy samples obtained from sufferers with out bone marrow involvement stained with CD271 (crimson), CD90 (pink), CD45 (grey) antibodies (high), and Imaris illustration of the 3D BM structure (backside). CD45+ hematopoietic cells (darkish inexperienced), CD45– CD271+ CD90– (crimson), CD45– CD271– CD90+ (pink), and CD45– CD271+ CD90+ MSCs (yellow). Photos have been acquired with 2 μm intervals to roughly 200- to 300 μm depths all through the BM tissue, 5–7 photographs per pattern.
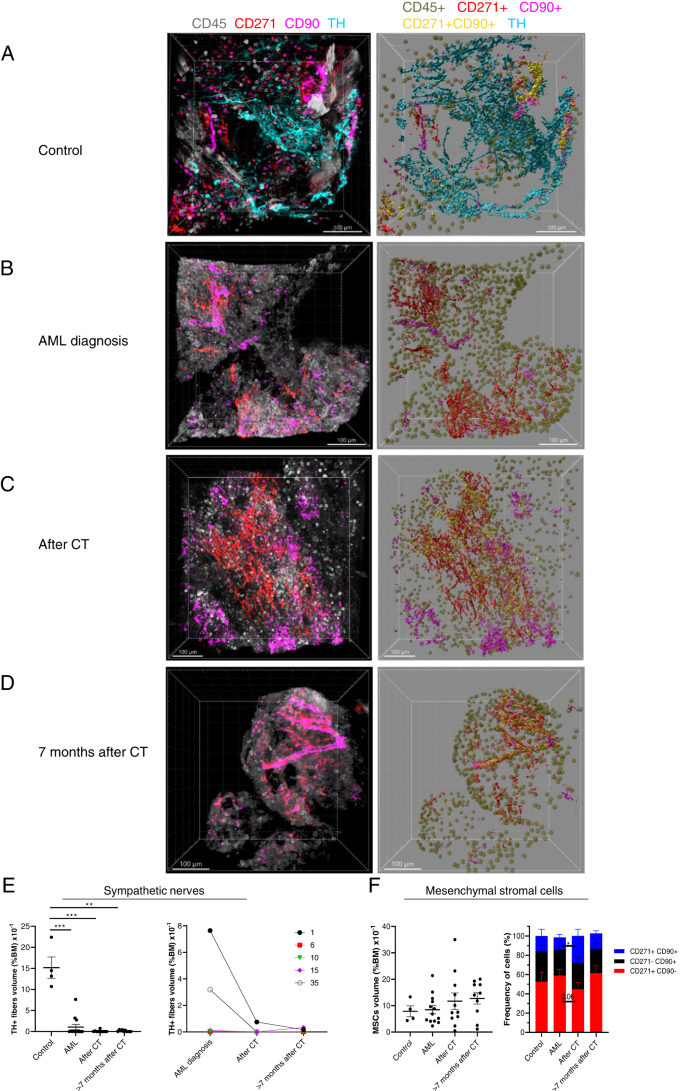
To analyze the distribution of SNFs in human BM, we used tyrosine hydroxylase (TH) staining as a marker for purposeful SNFs [8, 9] (Fig. 2A; Fig. S2 and Suppl. Video 2). We discovered that TH+ SNFs in human BM don’t present a corkscrew-shaped look as a result of wrapping round blood vessels prefer it was proven in murine BM [5, 7, 8, 13] however are distributed irregularly within the parenchyma (Fig. 2A and Fig. S2), indicating that findings and conclusions drawn from analysis carried out on mice will not be uniformly translated to human BM area of interest as a result of inherent organic variations between the 2 species.
A–D Authentic consultant z-stack confocal photographs of BM biopsy samples from A management sufferers’ BM, sufferers B at major AML prognosis, C 15–21 days after CT, and D > 7 months after CT (left). The correct panels show Imaris extracted photographs: CD45+ hematopoietic cells (darkish inexperienced), TH+ SNFs (cyan), CD45– CD271+ CD90– (crimson), CD45– CD271– CD90+ (pink), and CD45– CD271+ CD90+ MSCs (yellow). Samples have been stained with anti-TH (cyan), CD271 (crimson), CD90 (pink), and CD45 (grey). Photos have been acquired with 2 μm intervals to roughly 200- to 300 μm depths all through the BM tissue, 5–7 photographs per pattern. E Quantification of SNFs in BM from management sufferers (n = 4), from sufferers at AML prognosis (n = 13), after CT (n = 11), and > 7 months after CT (n = 10) (left). The correct panel represents SNF share in BM (ratio between the SNF quantity and complete quantity from all footage for every affected person x 100 %) over time for a similar affected person (n = 5). F Quantification of MSC densities (the ratio between the MSC quantity and complete quantity from all footage for every affected person x 100 %) in management BM (n = 4), from sufferers at AML prognosis (n = 13), after CT (n = 11), and > 7 months after CT (n = 10) (left). Composition of CD45– CD271+ CD90+, CD45– CD271– CD90+, and CD45– CD271+ CD90– MSCs in human BM (proper). Every dot represents a single affected person pattern. Information represented as imply +/– S.E.M. *p < 0.05, *p < 0.01, ***p < 0.001 decided by unpaired Mann–Whitney take a look at.
Subsequent, we analyzed whether or not AML growth and CT change the density of MSCs and TH+ SNFs in sufferers’ BM. We collected trephine biopsy samples throughout routine diagnostic procedures from 25 AML sufferers (age: median = 64 years (vary: 22–81), intercourse: male = 14, feminine = 11, ELNRisk 2017 classes: opposed = 16, intermediate = 2, favorable = 7) (Suppl. Desk 1). The density of TH+ SNFs was considerably diminished within the BM of AML sufferers already on the major prognosis in comparison with controls (Fig. 2A–E and Fig. S3). The density of residual TH+ SNFs didn’t correlate with neither age nor blast frequency on the major prognosis and was dramatically diminished even within the BM of sufferers with low blast frequency (10–20 %) (Fig. S4A, B). Nonetheless, we seen that 3 out of 6 sufferers from favorable or intermediate threat classes had greater TH+ SNF density (2.7–7.6 × 10−1 % BM quantity) in contrast with these from the opposed class (in all samples under 0.01 × 10−1 % BM quantity) (Fig. S4C and Suppl. Desk 1). Importantly, we discovered that the medicine used for induction CT (Suppl. Desk 1) scale back the density of TH+ SNFs in BM even additional in comparison with major prognosis samples (Fig. 2B–E; Fig. S3 and Suppl. Desk 1), which is in settlement with murine fashions of cytotoxic remedy with vincristine and cisplatin [7]. Furthermore, TH+ sympathetic fibers didn’t restore even seven months after CT (Fig. 2D, E and Fig. S3).
It has been proven that sympathetic neuropathy in BM induces modifications in MSC quantity in murine leukemia fashions [8, 9]. Nonetheless, we discovered that MSC inhabitants density and composition weren’t modified within the BM of AML sufferers regardless of the extreme discount in sympathetic innervation in comparison with controls at any analyzed time factors (Fig. 2A–F and Fig. S5).
Due to this fact, for the primary time in human biopsy materials, we visualized MSCs and TH+ SNFs in management and AML BM. Our research reveals the detrimental and chronic impact of invasive leukemic progress on the sympathetic neural part of the human BM area of interest, which might result in long-lasting hematological dysfunction. Since sympathetic reinnervation after strong organ transplantation has been related to improved organ operate [14], additional investigation must be carried out to validate whether or not the restoration of purposeful TH+ SNFs generally is a therapeutic aim in sufferers with AML.