Aberrant activation of HGF/MET and canonical Wnt axes happens in DNPC cells
To realize in-depth perception for mobile traits of DNPC, we analyzed transcriptomics of human CRPC samples12, defining them as AR pathway lively PCa (ARPC), DNPC, and neuroendocrine PCa (NEPC) as beforehand reported13. A big lower of AR activated applications by measuring the expression of AR downstream goal gene signatures revealed in each DNPC and NEPC in comparison with ARPC samples (Fig. 1a and Supplementary Fig. 1a). Gene Set Enrichment Evaluation (GSEA) utilizing a pre-ranked gene listing from differentially expressed genes (DEGs), with a |log2 fold change | > 1 and adjusted P-value < 0.05, additional recognized a big enrichment within the down-regulation of androgen-response pathways in DNPC and NEPC compared to ARPC samples (Fig. 1b and Supplementary Fig. 1b). Nonetheless, important upregulation of HGF/MET and canonical Wnt signaling downstream goal genes was noticed solely in DNPC by evaluating transcriptomic adjustments with ARPC, however not in NEPC with ARPC samples (Fig. 1a). Accordingly, GSEA confirmed a big enrichment within the upregulation of HGF/MET and Wnt/β-catenin signaling pathways utilizing the DEGs from DNPC versus ARPC (Fig. 1b and Supplementary Fig. 1c), however not NEPC versus ARPC cells (Supplementary Fig. 1b). Correlation analyses additional demonstrated a big inverse correlation between activation of AR and HGF/MET downstream targets in DNPC samples, straight supporting the above GSEA knowledge (Fig. 1c, d). A big constructive correlation between elevated expression of HGF/MET and Wnt/β-catenin downstream targets was additionally noticed in DNPC in addition to ARPC samples (Fig. 1c, d). Consultant photos of immunohistochemical analyses (IHC) confirmed E-cadherin constructive and synaptophysin (SYN) unfavorable staining in PCa cells of each naive major and ABI- and ENZ-treated CRPC affected person samples. Nonetheless, lack of typical nuclear AR staining together with constructive phosphorylated MET (pMET) and cytoplasmic β-catenin staining was noticed in adjoining sections of the above CRPC tissues however not in major PCa samples (Fig. 1e, please see the “Strategies” part), reaffirming activating MET and β-catenin signaling pathways correlated with lowered nuclear AR expression in these human CRPC cells. To realize in-depth perception into dysregulation of MET and Wnt/β-catenin axes throughout CRPC improvement, we analyzed single-cell RNA-sequencing (scRNA-seq) datasets derived from human major PCa and mCRPC14. After integrating the samples, epithelial cells had been extracted and re-clustered, and comparable cell clusters had been recognized and verified with NE and AR scores in each major PCa and mCRPC samples (Supplementary Fig. 1d–h). The luminal epithelial cell clusters 6–8 (LE6-8), predominately existed in mCRPC samples with considerably decrease AR scores than different clusters primarily recognized in major PCa samples (Supplementary Fig. 1h). Violin expression plots additionally confirmed low expression of AR in these clusters (Supplementary Fig. 1i). In distinction, elevated expression of MET, CTNNB1, and their downstream targets, together with SERPINB1, CLDN1, DKK1, CCND1, MMP7, and PLAUR, respectively, was detected in two mCRPC predominant clusters, LE7 and LE8 (Supplementary Fig. 1i). These scRNA-seq knowledge present high-resolution depictions to straight hyperlink lowered AR expression and exercise with elevated activation of MET and CTNNB1 signaling pathways in mCRPC cells. Taken collectively, our knowledge recognized a correlation between elevated HGF/MET and Wnt/β-catenin activation with lowered AR signaling pathways, which uncovers a definite mechanism for DNPC improvement.
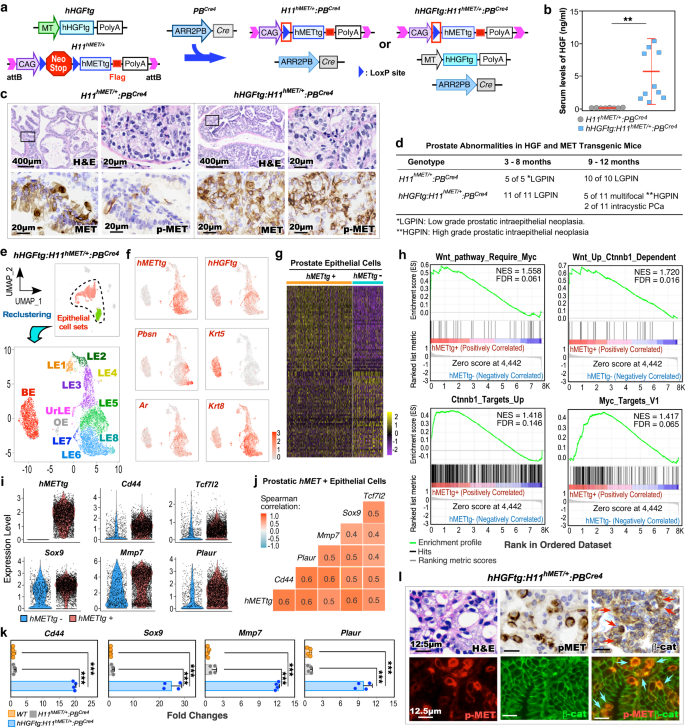
a Heatmap exhibiting rating of indicated gene signatures or expression profile of indicated genes throughout human metastatic castration-resistant prostate most cancers (mCRPC) samples obtained from SU2C/PCF RNA-seq datasets (2019) at cBioPortal12,13 (ARPC AR-active prostate cancers, n = 177; NEPC neuroendocrine prostate cancers, n = 13; DNPC double-negative AR-null NE-null prostate cancers, n = 14). Scores of AR- or NE-associated gene signature are proven within the prime panel primarily based on the earlier research13. HGF/MET pathway-associated genes and Wnt/β-catenin downstream goal genes are listed within the center and backside panel, respectively. Colours mirror the extent of gene signature rating or expression. See “Strategies” part. b Gene Set Enrichment Evaluation (GSEA) enrichment plots of pre-ranked gene listing from differentially expressed genes (DEGs) evaluating DNPC to ARPC samples. NES normalized enrichment rating, FDR false discovery charge. See Supplementary Knowledge 1. c Heatmap of pairwise Spearman correlation between the indicated gene signatures in indicated CRPC samples. Numbers point out correlation coefficient. Colours mirror the correlation coefficient worth. d Scatter plots displaying the mRNA expression z-scores of indicated gene signatures in indicated CRPC samples. The pink strains present affiliation between the gene signatures and r signifies Spearman’s correlation coefficient. e Consultant photos of immunohistochemistry staining utilizing the indicated antibodies on tumor tissues from naive major prostate adenocarcinoma (PCa, n = 5) and androgen deprivation remedy (ADT)-treated CRPC sufferers (n = 6, please additionally see the “Materials” part). AR androgen receptor, NE neuroendocrine, E-CAD E-cadherin, SYN synaptophysin, pMET phosphorylated MET, β-cat β-catenin. Consultant photos from three unbiased experiments with related outcomes are displayed for every micrograph. Scale bars, 25 μm.
Reciprocal activation of HGF/MET signaling prompts canonical Wnt signaling in prostate oncogenesis
To realize a direct and mechanistic perception into the activation of HGF/MET to induce Wnt/β-catenin signaling in DNPC pathogenesis, we generated hHGFtg:H11hMET/+:PBCre4 double transgenic mice, known as DoubleTg (Fig. 2a). These mice have concomitant ubiquitous expression of human HGF transgene (hHGFtg) and conditional expression of human MET transgene (hMETtg) to imitate the paracrine interplay between aberrant HGF and MET activation as noticed in PCa sufferers15,16,17. Elevated HGF ranges had been noticed particularly in sera of DoubleTg in comparison with H11hMET/+:PBCre4 mice (Fig. 2b). Accordingly, strong pMET was detected in atypical cells inside prostatic intraepithelial neoplasia (PIN) lesions of DoubleTg mice however not in H11hMET/+:PBCre4 mice regardless of the expression of transgenic human MET showing in atypical cells of each samples (Fig. 2c). Moreover, no expression of MET and pMET was detected in prostate tissues of wild-type controls (Supplementary Fig. 2a). Based mostly on the rules offered by “the New York Pathology Panel”18, we recognized pathological lesions representing low and high-grade PIN (LGPIN, HGPIN), or intracystic adenocarcinomas in prostate tissues of 3-, 6-, or 9-month-old DoubleTg mice, respectively, (Fig. second and Supplementary Fig. 2b), demonstrating reciprocal activation of MET by way of HGF selling PCa improvement. To evaluate the regulatory mechanism underlying the reciprocal activation of HGF/MET in prostate tumorigenesis, we carried out scRNA-seq analyses utilizing pathologically confirmed prostate tissues containing HGPIN and PCa lesions from DoubleTg mice. After qc and filtering procedures, cell units had been seen in Uniform Manifold Approximation and Projection (UMAP) plots19,20,21 (Fig. 2e and Supplementary Fig. 2c–g). To evaluate the in-depth molecular adjustments in prostatic tumor epithelia, we extracted epithelial cells and re-clustered them (Supplementary Fig. 2h–j). The expression of hMETtg appeared primarily in LE clusters (Fig. 2f and Supplementary Fig. 2k). The DEGs, which confirmed in additional than 5% of cells with an adjusted P-value < 0.05 and |common log2 fold change| > 0.1, had been recognized utilizing a Wilcoxon Rank Sum take a look at by evaluating hMETtg+ and hMETtg− epithelial cells (Fig. 2g). GSEA utilizing the above pre-ranked DEGs recognized a big enrichment in upregulation and activation of Wnt/β-catenin and associated Myc activation pathways (Fig. 2h), in addition to pathways straight associated to prostate tumorigenesis (Supplementary Fig. 2l). A considerably greater expression of hMETtg and β-catenin downstream targets, Cd44, Sox9, Tcf7l2, Mmp7, and Plaur was additionally recognized in hMETtg+ than hMETtg− epithelial cells on violin expression plots (Fig. 2i). Spearman gene-gene correlation evaluation additional demonstrated a big correlation of the hMETtg with these β-catenin targets (Fig. 2j). Quantitative reverse transcription-PCR (qRT-PCR) analyses verified the upper expression of Wnt/β-catenin targets, Cd44, Sox9, Mmp7, and Plaur in RNA samples ready from PCa tissues of DoubleTg mice than these from controls (Fig. 2k). IHC analyses revealed a rise in cytoplasmic and nuclear β-catenin expression in PCa cells with constructive pMET expression in adjoining tissue sections (Purple arrows within the prime panel, Fig. 2l) however not in prostate samples of wild-type management mice (the left panel, Supplementary Fig. 2m). Accordingly, co-immunofluorescence (co-IF) analyses additionally confirmed overlay of cytoplasmic and nuclear β-catenin with pMET staining in these prostate tissue specimens (blue arrows within the backside panel, Fig. 2l), and there’s a important improve in double constructive cells in comparison with prostate tissues from the wild-type mice (the best panel, Supplementary Fig. 2m, n). These strains of experimental proof implicate a regulatory mechanism by which activating HGF/MET signaling induces canonical Wnt axes in the course of the course of PCa improvement.
a Schematic of the human HGF (hHGFtg) and MET (H11hMET) transgenes, and PBCre4 alleles, proven in relation to the mating technique. b Serum HGF ranges of H11hMET/+:PBCre4 (n = 8) and hHGFtg:H11hMET/+:PBCre4 (n = 10) mice. c Consultant photos of hematoxylin-eosin (H&E) and immunohistochemistry (IHC) staining utilizing the indicated antibodies on adjoining prostate tissues from the indicated mice. Scale bars, 400 μm, 20 μm. d Desk summarizing the pathological abnormalities within the prostates of H11hMET/+:PBCre4 and hHGFtg:H11hMET/+:PBCre4 mice on the indicated age. e Uniform Manifold Approximation and Projection (UMAP) plots presenting complete cells (n = 9236) with highlighting prostatic epithelial cells (each inexperienced and pink cell clusters) from hHGFtg:H11hMET/+:PBCre4 mice, and epithelial cells (n = 7286) being additional sub-clustered, re-clustered, and labeled by epithelial cell cluster (backside). The dotted line delineated the prostatic epithelial cells. BE basal epithelial cells, LE luminal epithelial cells, UrLE urethral epithelial cells, OE different epithelial cells. f Gene expression UMAP plots for the indicated genes in epithelial cells (n = 7286). Coloration depth signifies the scaled expression degree. g Heatmap exhibiting DEGs between hMETtg+ and hMETtg− epithelial cells. See Supplementary Knowledge 2. h GSEA plots exhibiting the constructive enrichment of the indicated gene units evaluating hMETtg+ and hMETtg− cells. NES normalized enrichment rating, FDR false discovery charge. i Violin plots visualizing the expression ranges of hMETtg and Wnt downstream goal genes in hMETtg+ (n = 4533) and hMETtg− (n = 2753) epithelial cells. Black dots correspond to particular person epithelial cells. j Heatmap of pairwise Spearman correlation between the indicated genes in hMETtg+ epithelial cells. Colours mirror the correlation coefficient worth. ok qPCR evaluation of the indicated genes proven as fold change in indicated mouse prostate tissues from 4 organic replicates. l Consultant photos of H&E, IHC, and immunofluorescence staining (IF) utilizing indicated antibodies on adjoining sections from hHGFtg:H11hMET/+:PBCre4 mice. Purple and blue arrows point out nuclear β-catenin and co-overlay of pMET with nuclear β-catenin, respectively. Consultant photos with constant outcomes from three organic replicates are proven. Scale bars, 12.5 μm. In b and ok, knowledge are imply ± s.d. **P < 0.01, ***P < 0.001; Unpaired two-tailed t-tests. See supply knowledge and the precise P values within the Supply Knowledge file.
Aberrant HGF/MET and canonical Wnt activation develops strong invasive and metastatic PCa with double-negative mobile properties
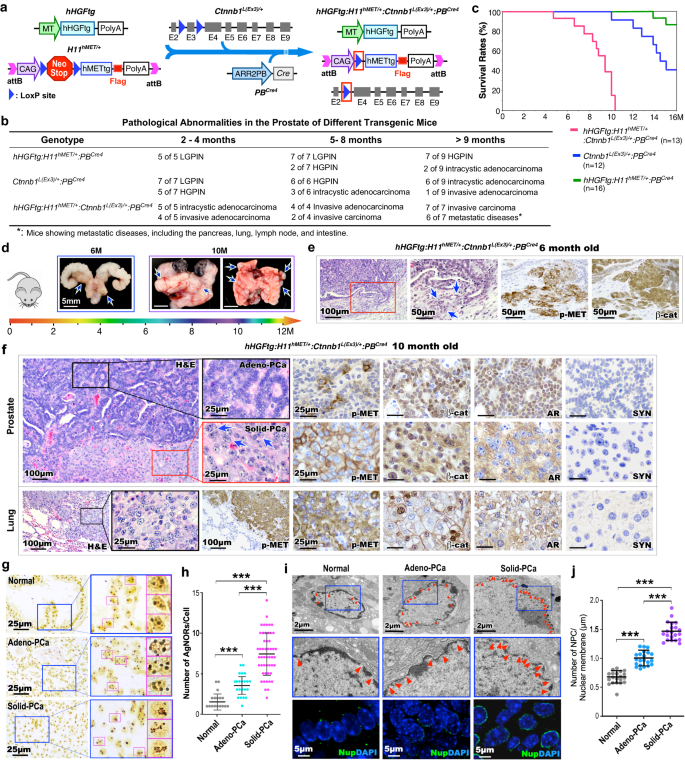
To straight study the organic penalties of HGF/MET and canonical Wnt signaling co-activation in prostate epithelia, we developed the hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mouse mannequin, known as TripleTg, by which each the human MET transgene and stabilized β-catenin are expressed in prostatic epithelia together with ubiquitous human HGF transgene expression to imitate observations from scientific DNPC samples (Fig. 3a). Elevated ranges of HGF had been additionally detected in sera of TripleTg mice (Supplementary Fig. 3a). Strong invasive prostate adenocarcinomas developed a lot earlier in TripleTg than DoubleTg, or stabilized β-catenin solely transgenic mice (Fig. 3b). Furthermore, metastatic tumor lesions at a number of organs revealed as early as at 9 months of age in TripleTg mice (Fig. 3b), akin to the shortest survival charge examine with different management mice (Fig. 3c). The sooner rising and extra aggressive tumor behaviors had been recognized grossly in consultant 6-, and 10-month-old TripleTg mice, that includes a number of tumors in several prostatic lobes, native invasion into seminal vesicles, and a number of metastatic tumor lesions (Fig. 3d). Pathological examination of 6-month-old TripleTg mice confirmed invasive adenocarcinoma lesions with constructive staining for each pMET and nuclear β-catenin within the malignant prostate tissue (Fig. 3e). Analyses of prostate tissues from 10-month-old TripleTg mice recognized a collection of progressive tumor lesions coexisted in the identical prostate tissues. They included lesions with glandular traits and well-differentiated adenocarcinomas, just like human Gleason Grade 3–4 prostate carcinomas, termed “Adeno-PCa” on this research, in addition to lesions with poorly differentiated traits containing considerable flippantly eosinophilic cytoplasm and pleomorphic nuclei with out distinct gland formation, akin to Gleason Grade 5 prostate carcinomas, termed “Stable-PCa” (Fig. 3f). It ought to be famous that each Adeno- and Stable-PCa are phrases solely used to indicate the phenotypes on this research. Stable-PCa cells moreover displayed irregular nucleolar traits with outstanding and infrequently a number of nucleoli (blue arrows, Fig. 3f). Constructive pMET and nuclear β-catenin staining had been noticed in each kinds of tumor cells. Nonetheless, typical nuclear AR staining solely appeared in Adeno-PCa cells. In distinction, Stable-PCa cells confirmed noticeable cytoplasmic however no or very weak nuclear AR staining (Fig. 3f), presenting with related mobile properties to these noticed in human DNPC (Fig. 1e). Each kinds of tumor cells additionally revealed constructive staining for CK8 and E-cadherin however had been unfavorable for SYN verifying their epithelial properties (Fig. 3f and Supplementary Fig. 3b). Evaluation of lung metastatic tumor lesions confirmed related cell traits as noticed in poorly differentiated Stable-PCa, that includes flippantly eosinophilic cytoplasm and irregular nuclei with distinctly everlasting nucleoli (Fig. 3f). Constructive staining for pMET and nuclear β-catenin, with no nuclear AR staining was additionally detected in lung metastatic tumor cells (Fig. 3f). Gross and histological examination of metastatic tumor lesions from gut and spleen tissues revealed very related tumor cell traits as discovered within the lung metastatic lesions (Supplementary Fig. 3c). Measuring the expression of AR and SYN in human PCa (Fig. 1e) and TripleTg mouse prostate PCa tissues (the center panel, Fig. 3f) confirmed important discount of nuclear AR expression in each PCa cells in human tissues remoted from sufferers handled with ABI and ENZ and Stable-PCa cells from TripleTg mice (Supplementary Fig. 3d). Taken collectively, improvement of aggressive and metastatic PCa lesions in TripleTg mice demonstrates a big function for co-activation of HGF/MET and canonical Wnt axes in selling PCa development. Particularly, the shortage of nuclear AR expression in poorly differentiated Stable-PCa cells of TripleTg mice corroborates the mobile traits noticed in human DNPC cells, additional suggesting a regulatory function of aberrant activation of HGF/MET and Wnt axes in PCa development and DNPC improvement.
a Schematic of producing completely different transgenic mice as indicated above. b Desk summarizing the pathological abnormalities within the prostates from completely different genotype mice. c Kaplan–Meier survival curves for the indicated transgenic mice. d Consultant gross photos of prostate tumor tissues with seminal vesicles and urinary bladders and lung tissues from both 6- or 10-month-old hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. Blue arrows point out major tumor or metastasis loci. e Consultant photos of H&E and IHC staining utilizing the indicated antibodies on adjoining prostate tissues from hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice at 6 months of age. Blue arrows level to invasive lesions. f Consultant photos of H&E and IHC staining utilizing the indicated antibodies on adjoining prostate (prime) and lung (backside) tissues from 10- month-old hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. Blue arrows point out enlarged nucleoli. g Consultant photos of AgNOR stained prostate tissue sections containing regular prostatic glands, gland-forming prostate adenocarcinoma (Adeno-PCa), and strong prostate carcinoma (Stable-PCa) lesions in hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. Pink field highlights single nucleus. h Quantification of AgNOR quantity in particular person cells from the indicated loci. Numbers of AgNORs per cells had been measured for 50 cells in every picture, and a minimum of 5 photos from three organic replicates had been analyzed. i Consultant photos of transmission electron microscopy (prime) and immunofluorescent staining for Nups (NUP62, NUP98, and NUP214) detected by mAb414 antibody (backside) within the indicated loci from prostate tissues. Purple arrowheads level to nuclear pore complexes (NPC). Nuclei had been stained with DAPI. j Numbers of NPC per μm of nuclear membrane had been quantified, a minimum of 20 cells in every picture and 5 photos from three organic replicates had been analyzed. Consultant photos with constant outcomes from three biologically unbiased experiments are proven. Scale bars, 100 μm (e, f), 50 μm (e), 25 μm (f, g), 5 μm (i), 2 μm (i). In h and j, knowledge are represented as imply ± s.d. **P < 0.01; Unpaired two-tailed t-tests. Supply knowledge and the precise P values are offered within the Supply Knowledge file.
Rising nucleolar measurement and quantity, ensuing from elevated ribosome synthesis, are thought-about as hallmarks of aggressive tumor cells and are carefully correlated to poor prognosis22. To evaluate the nucleolar abnormalities recognized in PCa cells of TripleTg mice (Fig. 3f), we carried out AgNOR assay to look at silver-stained proteins related to the nucleolar organizer areas, AgNORs, within the nuclei. Considerably extra AgNORs revealed in Stable-PCa than Adeno-PCa, or regular prostatic epithelial cells, correlating to a bigger nucleolar space (Fig. 3g, h and Supplementary Fig. 3g). As a result of the dimensions and quantity of AgNORs mirror nucleolar and cell proliferative exercise of tumor cells23, these outcomes additional correlated with the fast-growing and aggressive traits of Stable-PCa cells. Utilizing the transmission digital microscopy (TEM) analyses, we recognized a lot bigger nuclear measurement in Stable-PCa than in Adeno-PCa cells and regular epithelial cells (Supplementary Fig. 3e, f). Inspecting scientific samples additionally confirmed a lot greater variety of AgNORs and bigger measurement of nucleoli in CRPC cells of ABI- and ENZ-treated affected person samples than naïve PCa cells (Supplementary Fig. 3h, i), demonstrating related mobile properties between mouse Stable-PCa and human DNPC cells.
As a result of the nuclear pore complexes (NPC) perform because the central mediators of nucleo-cytoplasmic transport, elevated numbers of NPC amplify the nuclear transport equipment to advertise tumor development, and ceaselessly happen in aggressive tumors24,25, together with PCa cells26. TEM analyses confirmed considerably extra NPC in Stable-PCa cells than Adeno-PCa and management epithelial cells (Fig. 3i, j). IF assays revealed elevated expression of nucleoporins (Nups), the structural elements of the NPC, in Stable-PCa cells compared to different management samples (Fig. 3i). Figuring out these a number of nuclear abnormalities in Stable-PCa cells straight helps their aggressive and metastatic tumor mobile traits and offers in-depth molecular and mobile adjustments induced by co-activating HGF/MET and Wnt signaling to advertise PCa development and DNPC improvement.
Aberrant HGF/MET and canonical Wnt activation induces nuclear exporting and ribosomal synthesis pathways to advertise PCa development and metastasis
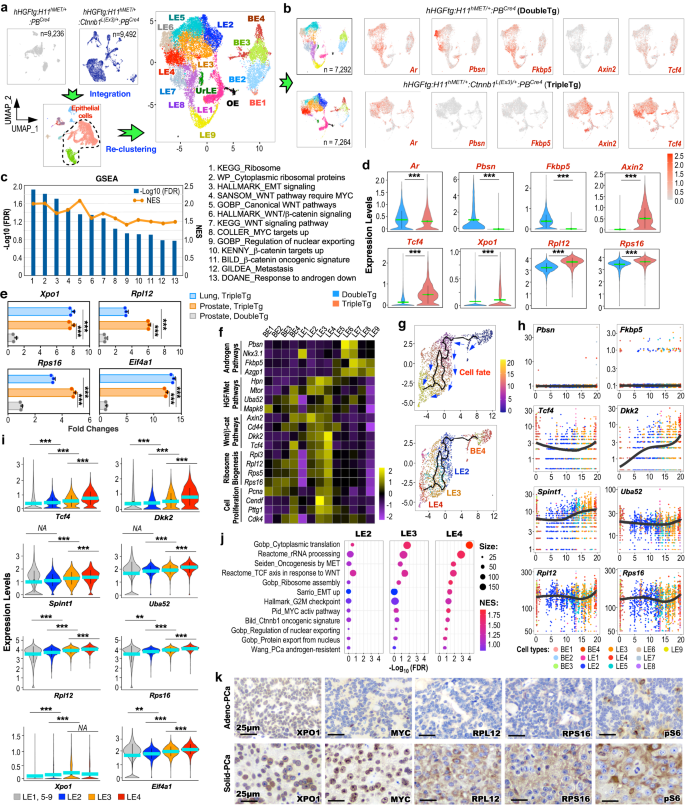
To know the regulatory function of HGF/MET and canonical Wnt axes in PCa development and metastasis, we carried out scRNA-seq analyses utilizing pathologically confirmed PCa tissues of TripleTg mice. The scRNA-seq samples from TripleTg and DoubleTg mice had been built-in, aligning 9 related cell subsets from each samples primarily based on their transcriptomic profiles19 (Supplementary Fig. 4a–d). To realize excessive decision of the mobile properties, epithelial cells had been extracted from complete cells, and re-clustered to fifteen epithelial cell clusters following cell cycle regression (Fig. 4a and Supplementary Fig. 4e–h). UMAP expression plots confirmed related Ar expression in epithelial cells of TripleTg and DoubleTg mice, however with noticeable discount of AR-regulated gene, e.g. Pbsn and Fkbp5, expression, and elevated expression of β-catenin downstream targets, Axin2 and Tcf4, revealed in TripleTg samples compared to DoubleTg samples (Fig. 4b). GSEA utilizing DEGs of hMETtg+ cells of TripleTg versus these of DoubleTg samples additional confirmed down-regulation of androgen signaling pathways with upregulation of Wnt/β-catenin signaling pathways (Fig. 4c and Supplementary Fig. 4i), per our earlier remark of lowered nuclear AR and elevated stabilized β-catenin expression in Stable-PCa cells of TripleTg mice (Fig. 3f). Moreover, pathways associated to ribosome synthesis, nuclear exporting, and tumor metastasis had been additionally considerably enriched in TripleTg samples (Fig. 4c and Supplementary Fig. 4i). Violin expression plots confirmed lowered Ar expression, decreased expression of Pbsn and Fkbp5, and better expression of Axin2 and Tcf4, and Xpo1, a nuclear exporting regulator, Rpl12 and Rpl16, ribosomal proteins, in hMETtg+ cells of TripleTg mice than these of DoubleTg counterparts (Fig. 4d). Utilizing qRT-PCR analyses additional confirmed elevated expression of Xpo1, Rpl12, Rps16, and Eif4a1 in RNA samples from major prostate and lung metastatic tumor cells of TripleTg mice compared to these from PCa tissues of DoubleTg mice (Fig. 4e). These knowledge taken collectively reveal the regulatory function of HGF/MET and canonical Wnt co-activation in growing nuclear exporting and ribosomal synthesis pathways in hMETtg+ tumor cells of TripleTg mice, which additionally correlate to the nuclear abnormalities related to ribosomal synthesis noticed in Stable-PCa cells22,23.
a Particular person UMAP visualization of cells from DoubleTg (prime left, grey) and TripleTg (prime center, darkish blue) prostates, and built-in cells coloured by cell kind identities (backside). UMAP plot of epithelial cells re-clustered and labeled as BE, basal epithelial cells; LE, luminal epithelial cells; UrLE, urethral epithelial cells; OE, different epithelial cells (prime proper). b UMAP plots exhibiting indicated gene expression, separated by every genotype. Coloration depth signifies the scaled expression degree. c GSEA compares hMETtg+ cells from TripleTg versus DoubleTg mice. See Supplementary Knowledge 3. d Violin plots visualizing the indicated gene expressions in hMETtg+ cells (DoubleTg, n = 4417; TripleTg, n = 2144) of every genotype. e qPCR evaluation of the indicated genes proven as fold adjustments within the indicated tissues from three organic replicates. f Heatmap depicting common expression of genes related to indicated pathways in every cluster. g Pseudotime trajectory plots of hMETtg+ cells (n = 2144) from TripleTg mice, visualized on UMAP plots by pseudotime (prime) and cluster id (backside). h Linear pseudotime expression plots exhibiting dynamics of indicated gene expression over pseudotime in hMETtg+ cells from TripleTg mice. Dots correspond to particular person cells coloured by cluster id. i Violin plot visualizing the expression ranges of indicated genes from TripleTg mice (LE1, 5–9, n = 1934; LE2, n = 1165; LE3, n = 1666; LE4, n = 1096). j Bubble charts exhibiting GSEA by evaluating LE2, LE3, or LE4 versus different LEs in TripleTg pattern. Coloration and measurement of bubbles represents NES and weighted numbers of genes. See Supplementary Knowledge 4–6. ok Consultant photos of IHC utilizing indicated antibodies on adjoining sections from TripleTg mice. Consultant photos with constant outcomes from three organic replicates are proven. Scale bars, 25 μm. In d–i, knowledge are imply ± s.d. **P < 0.01 and ***P < 0.001, two-tailed Wilcoxon Rank Sum assessments (d, i), unpaired two-tailed t-tests (e). Inexperienced or blue bar signifies the imply worth (d, i). Supply knowledge and the precise P values are offered within the Supply Knowledge file.
Analyzing built-in epithelial cell clusters confirmed that BE4 and LE1–4 clusters had been predominant in TripleTg samples, however different clusters had been enriched in DoubleTg samples (Supplementary Fig. 4j). Decreased expression of AR downstream targets, however elevated expression of HGF/MET and Wnt/β-catenin downstream goal genes in addition to ribosomal associated genes had been primarily recognized in BE4 and LE1–4 clusters by transcriptomic analyses throughout the epithelial cell clusters (Fig. 4f and Supplementary Fig. 4k). Moreover, elevated expression of genes straight associated to cell proliferation had been noticed particularly in LE3 and LE4 clusters, offering the molecular foundation for the poorly differentiated and fast-growing tumor cells traits noticed in prostate tumors of TripleTg mice. Utilizing single-cell trajectory analyses by Monocle3, we assessed dynamic and in-depth transcriptomic adjustments governing tumor improvement and development in hMETtg+ cells of TripleTg mice27. As proven in pseudotime trajectory plots (Fig. 4g), BE4 cells act as a place to begin and additional differentiate and progress to luminal cell branches primarily constituting LE2, 3, and 4 clusters. Considerably low expression of Pbsn and Fkbp5, with steadily elevated expression of Tcf4 and Dkk2, Spint1 and Uba52, HGF/MET signaling downstream targets, and Rpl12 and Rps16, respectively, had been revealed by BE4 to LE2-4 cell clusters throughout pseudotime development (Fig. 4h). Violin expression plots additional confirmed steadily elevated expression of Tcf4, Dkk2, Spint1, Uba52, Rpl12, Rps16, Xpo1, and Eif4a1 by LE2, 3, and 4 clusters compared to different LE clusters of TripleTg samples (Fig. 4i). GSEA utilizing DEGs of LE2, 3, or 4 versus different LE clusters recognized a big enrichment within the signaling pathways associated to protein synthesis, translation, rRNA processing, HGF/MET and Wnt signaling activation, nuclear export, ribosome biogenesis, and epithelial-mesenchymal transition (EMT) activation (Fig. 4j). These knowledge present a dynamic, single-cell decision depiction of aberrant co-activation of HGF/MET and Wnt/β-catenin in elevating nuclear exporting, ribosome synthesis, and oncogenic pathways to advertise PCa development and DNPC improvement. In distinction, hMETtg+ cells from DoubleTg mice exhibited a unique trajectory destiny, beginning with BE1–3 cells and differentiating into luminal cell branches possessing LE5–9 cells (Supplementary Fig. 4l). Moreover, elevated expression of AR-regulated genes and lowered expression of HGF/MET and Wnt/β-catenin downstream targets in addition to Rpl12 and Rps16 revealed by luminal hMETtg+ cells of DoubleTg mice (Supplementary Fig. 4m). IHC analyses utilizing adjoining PCa tissues of TripleTg mice confirmed the upper expression of XPO1, MYC, RPL12, RPS16, and pS6 in Stable-PCa cells than Adeno-PCa cells (Fig. 4k), additional supporting the above scRNA-seq outcomes, and suggesting that Stable-PCa are derived from Adeno-PCa throughout tumor development in TripleTg mice. Provided that MYC is a grasp regulator of ribosome biogenesis28, figuring out elevated MYC expression in Stable-PCa cells by activating Wnt/β-catenin signaling outlines a regulatory mechanism for aberrant activation of XPO1, ribosomal biogenesis, and protein synthesis pathways to advertise PCa development and DNPC improvement. Analyzing scientific samples, we additionally recognized elevated expression of XPO1 and RPL12 in DNPC samples from ABI- and ENZ-treated sufferers (Supplementary Fig. 5a). A rise within the expression of XPO1 was additionally detected in two human mCRPC predominant clusters, LE7 and LE8 (Supplementary Fig. 5b)14, which has been proven to own activated HGF/MET and Wnt/β-catenin axes (Supplementary Fig. 1i). Analyses of current human CRPC datasets recognized considerably lowered AR downstream targets (AR rating) in DNPC compared to ARPC samples (Supplementary Fig. 5c)29. Accordingly, greater expression for HGF/MET downstream targets (HGF rating) and WNT/β-catenin downstream targets (WNT rating) was additionally revealed in DNPC samples. Considerably lowered AR expression and elevated XPO1 expression had been additional proven in DNPC compared to ARPC (Supplementary Fig. 5d). Taken in complete, these strains of experimental proof additional help that aberrant co-activation of HGF and Wnt/β-catenin signaling pathways improve XPO1 expression throughout DNPC improvement.
Aberrant activation of HGF and Wnt signaling will increase CRM1/XPO1 expression by elevated SP1 expression
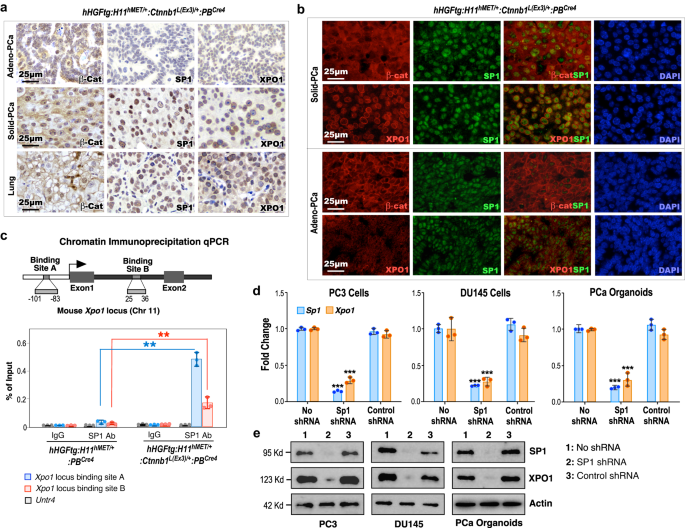
Figuring out elevated expression of XPO1 in poorly differentiated Stable-PCa cells in TripleTg mice suggests a regulatory function of Wnt/β-catenin signaling in PCa development and DNPC improvement. It has been proven that SP1 regulates Xpo1 transcription30 and β-catenin enhances SP1 transcriptional exercise by straight interacting and stabilizing the SP1 protein31. Utilizing IHC approaches, we first assessed the expression of stabilized β-catenin on SP1 and XPO1 expression in mouse PCa samples. Whereas the expression of cytoplasmic and nuclear β-catenin appeared in Adeno-PCa cells, outstanding nuclear β-catenin expression was noticed in each Stable-PCa and lung metastatic tumor cells of TripleTg mice (Fig. 5a). Accordingly, elevated expression of SP1 and peri-nuclear staining of XPO1 solely confirmed in each prostatic Stable-PCa and lung metastatic tumor cells however not in Adeno-PCa cells of TripleTg mice and PCa samples of DoubleTg mice in adjoining tissue sections (Fig. 5a and Supplementary Fig. 5e). Co-IF analyses additional confirmed that elevated expression of SP1 was overlaid with intensive stabilized β-catenin expression in Stable-PCa cells however not in Adeno-PCa cells of TripleTg mice, and PCa cells of DoubleTg mice (Fig. 5a, b and Supplementary Fig. 5f). Furthermore, predominant peri-nuclear staining of XPO1, overlaying with the nuclear SP1, was particularly seen in Stable-PCa cells however not in Adeno-PCa cells and in PCa cells of DoubleTg mice (Fig. 5a, b and Supplementary Fig. 5f). These knowledge elucidate the regulatory function of stabilized β-catenin on growing SP1 and XPO1 expression in Stable-PCa cells. A number of SP1 binding websites had been recognized inside the promoter area of the Xpo1 gene and, by these websites, SP1 can regulate Xpo1/Crm1 transcription in reworked tumor cells30. Utilizing chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analyses, we straight examined the binding of SP1 to the promoter of Xpo1 thereby activating its transcription. Particular occupancy of SP1 was recognized within the SP1 binding websites inside each the promoter and enhancer areas of the Xpo1 gene locus, however not the Untr4 locus, used as a unfavorable management, in PCa cells remoted from TripleTg mice, and likewise not in PCa cells remoted from DoubleTg mice (Fig. 5c)30. Utilizing knockdown approaches, we additional demonstrated that particular discount of SP1 expression considerably diminishes the expression of XPO1 transcripts and proteins in two human prostate most cancers cell strains, DU145 and PC3, in addition to in prostate organoid cultures derived from PCa tumors of TripleTg mice (Fig. 5d, e). These strains of experimental proof reveal the regulatory function of Wnt/β-catenin signaling activation in enhancing SP1-mediated Xpo1 expression in PCa cells. Provided that dysregulation of XPO1 straight contributes to tumor improvement, development, and most cancers drug resistance32,33,34, our findings implicate a regulatory mechanism underlying aberrant Wnt/β-catenin activation to advertise PCa development, hormone refractoriness, and DNPC improvement by enhancing SP1-regulated XPO1 activation.
a Consultant photos of IHC staining utilizing the indicated antibodies on completely different prostate lesions (prime and center) and lung (backside) tissues from 10-month-old hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. b Consultant photos of co-IF staining of SP1 with β-catenin or XPO1 in prostate tissues of hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. c The scheme of the Xpo1 gene locus and ChIP-qPCR analyses on SP1 binding websites utilizing indicted antibodies. d qPCR evaluation of SP1 and XPO1 proven as fold change in PC3 cells, DU145 cells, and organoids derived from dissected prostate cells of hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. e Immunoblotting of cell lysates after indicated shRNA remedy exhibiting protein expression of SP1, XPO1, and Actin in PC3, DU145, and prostate organoid cells derived from PCa tumors of hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. Actin was used as an inner customary. In c and d, knowledge are represented as imply ± s.d. of three organic replicates. **P < 0.01, ***P < 0.001; Unpaired two-tailed t-tests. In a, b, and e, consultant photos with constant outcomes from three organic replicates are proven. Scale bars, 25 μm. Supply knowledge and the precise P values are offered within the Supply Knowledge file.
Aberrant activation of XPO1 and ribosomal synthesis pathways converts PCa mobile properties and promotes androgen-independent development
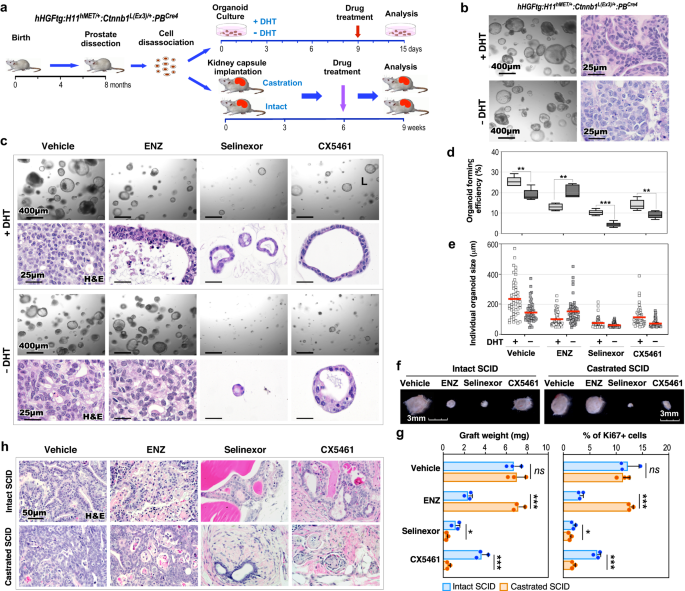
Our knowledge from scRNA-seq and different approaches recognized aberrant XPO1 and ribosomal synthesis activation in Stable-PCa cells from TripleTg mice. Utilizing each organoid tradition and in vivo graft approaches, we straight assessed these abnormalities in regulating androgen-independent PCa cell development (Fig. 6a). Intriguingly, prostatic tumor organoids that had been derived and developed from dissected PCa cells of TripleTg mice confirmed the flexibility to develop in tradition both with or with out dihydrotestosterone (DHT) (Fig. 6b). Histological analyses recapitulated related mobile traits of Adeno-PCa in organoids cultured with DHT, nonetheless, their counterparts cultured with out DHT confirmed much less differentiated tumor traits, just like Stable-PCa cells (Fig. 6b). Organoids cultured with DHT confirmed constructive nuclear AR staining however these cultured with out DHT confirmed no nuclear AR staining with correspondingly extra intense peri-nuclear staining of XPO1 and stronger cytoplasmic staining of RPS16, whereas each samples revealed constructive E-cadherin staining (Supplementary Fig. 5g). The AR antagonist, ENZ demonstrated important inhibition of tumor organoid development solely in samples cultured with DHT however not in these cultured with out DHT (Fig. 6c). Nonetheless, Selinexor or CX5461, a XPO1 or ribosome inhibitor, respectively, displayed a big repressive impact on organoid development in each samples cultured with or with out DHT (Fig. 6c). Measuring common sizes of particular person organoids and the organoid forming effectivity additional affirmed the inhibitory results of ENZ, Selinexor, and CX5461 (Fig. 6d, e). The impact of the above inhibitors on PCa development was additional examined utilizing in vivo tissue grafting assays (Fig. 6a). The dimensions and weight of prostatic tumor grafts handled with ENZ had been considerably smaller and fewer than vehicle-treated counterparts in intact hosts, however no completely different in castrated mice (Fig. 6f, g), confirming the androgen-independent development capability of PCa cells developed from TripleTg mice. Grafts handled with Selinexor had been considerably smaller and weighed lower than vehicle-treated samples in each intact and castrated hosts, and CX5461 confirmed a better inhibitory impact in castrated hosts than in intact hosts (Fig. 6f, g). Histological analyses confirmed much less differentiated tumor traits in vehicle-treated grafts from castrated host compared to these from intact hosts (Fig. 6h). Pathological adjustments just like ADT-induced tumor regression had been exhibited in ENZ-treated grafts from intact hosts, and no tumor lesions appeared in Selinexor or CX5461-treated grafted samples (Fig. 6h). Measuring the variety of Ki67+ cells within the above samples correlated with the results of the completely different inhibitors as proven in each gross and pathological analyses (Fig. 6g). Taken collectively, the above knowledge reaffirm the promotional function of XPO1 and ribosomal biogenesis activation in androgen-independent PCa development, offering extra experimental proof for focusing on these oncogenic pathways to forestall PCa development, and DNPC improvement.
a Schematic illustration of the experimental design for the organoid tradition and kidney capsule transplantation assays. Organoids derived from dissected prostate cells of hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice had been developed after which handled with automobile and completely different inhibitors within the presence or absence of DHT for six days. Intact and castrated SCID host mice transplanted with dissected prostate cells of hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice had been administrated with automobile and completely different inhibitors. See “Strategies” part. b Consultant photos of brightfield and H&E staining for organoids within the presence or absence of DHT. c Consultant photos of brightfield and H&E staining for organoids with indicated remedies. d Quantification of organoid forming effectivity exhibiting the proportion of organoids above 50 μm diameter per complete cells seeded at day 0 in a properly. The middle line represents the median worth, the field borders symbolize the decrease and higher quartiles (25% and 75% percentiles, respectively), and the ends of the underside and prime whiskers symbolize the minimal and most values, respectively, for 5 unbiased samples over three organic replicates. e Quantification of particular person organoid measurement. Organoids per remedy group (n = 50) examined over three unbiased experiments. The middle pink bar signifies the imply worth in every group. f–h Consultant picture for gross (f) or H&E staining (h) of xenografts with the indicated remedies. Weights of xenografts (n = 3; left) and quantification of Ki67+ cells per complete cells (proper) from teams handled as indicated (g). Knowledge are represented as imply ± s.d. of three organic replicates. Consultant photos from three organic replicates are proven. In d and g, *P < 0.05, **P < 0.01, ***P < 0.001; Unpaired two-tailed t-tests. Supply knowledge and the precise P values are offered within the Supply Knowledge file.
Aberrant activation of HGF/MET and Wnt axes promotes mCRPC improvement
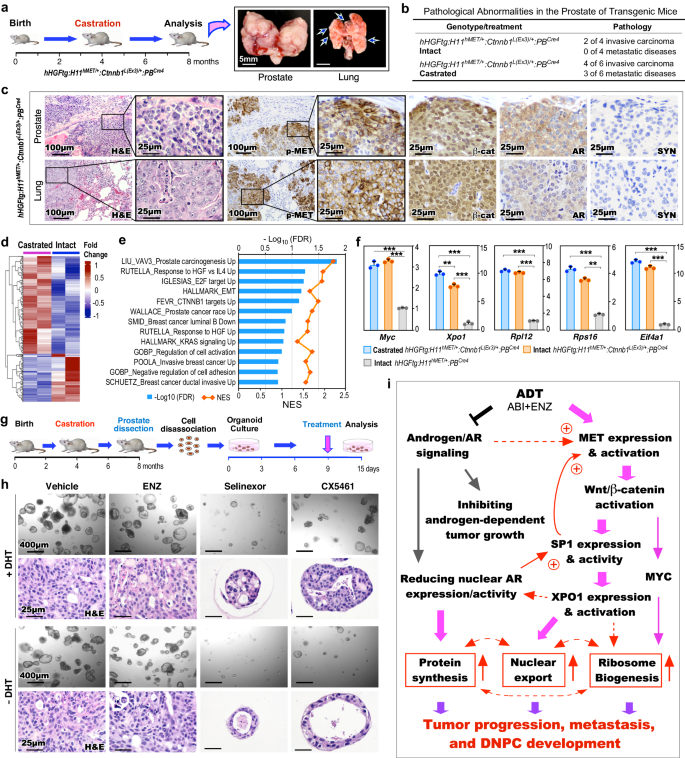
Given present ADT straight induces DNPC improvement, we examined the impact of co-activation of HGF/MET and Wnt/β-catenin in ligand-independent PCa development, development, and mCRPC improvement. TripleTg mice had been castrated at 4 months and analyzed at 8 months of age (Fig. 7a). A number of invasive tumor lesions occurred each domestically and on the distant websites in castrated mice in comparison with age- and genotype-matched intact counterparts (Fig. 7a, b). Histological analyses recognized poorly differentiated tumor lesions in each major and metastatic tumor samples, sharing very related mobile traits as noticed in Stable-PCa cells (Fig. 7c). Particular expression of pMET and nuclear β-catenin with a scarcity of nuclear AR and SYN expression was noticed in each prostate and lung metastatic tumor cells (Fig. 7c), confirming the double-null cell properties of mCRPC in castrated TripleTg mice. To realize in-depth perception into transcriptomic adjustments induced by castration, we analyzed bulk RNA-sequencing (RNA-seq) samples ready from microscopically confirmed PCa tissues of each intact and castrated TripleTg mice (Fig. 7d). GSEA utilizing pre-ranked gene lists from the above DEGs between castrated and intact samples revealed important enrichment in pathways associated to PCa oncogenesis, together with HGF, E2F, EMT, β-catenin, and KRAS activation in addition to tumor invasion and development (Fig. 7e). Outcomes from qRT-PCR analyses confirmed greater expression of Myc, Xpo1, Rpl12, Rps16, and Eif4a1 in each intact and castrated PCa samples from TripleTg mice than these from DoubleTg mice. A big improve in Xpo1 expression was noticed in castrated versus intact samples from TripleTg mice (Fig. 7f), reaffirming aberrant activation of XPO1 in PCa development and DNPC improvement. Constructive staining for XPO1, RPL12, RPS16, and pS6 proteins was additionally noticed in each major prostate and lung metastatic tumor cells developed in castrated TripleTg mice (Supplementary Fig. 6a). Utilizing prostatic organoid tradition organoids, we additional assessed the inhibition of XPO1 and ribosomal biogenesis pathways in CRPC cells from TripleTg mice (Fig. 7g). Related measurement and variety of organoids developed in tradition circumstances both with or with out DHT, and vehicle- and ENZ-treated organoids cultured with or with out DHT confirmed no important distinction (Fig. 7h). Nonetheless, Selinexor- or CX5461-treated organoids appeared considerably decreased in quantity and measurement in comparison with vehicle-treated samples, whereas the previous appeared stronger than the later (Fig. 7h). Quantifying common sizes of organoids, their forming effectivity, and percentages of Ki67+ cells in organoids confirmed the numerous inhibitory impact in Selinexor- or CX5461-treated samples compared to vehicle-treated counterparts (Supplementary Fig. 7b–d). These knowledge additional verify the impact of XPO1 and ribosomal synthesis pathway inhibitors on CRPC development, implicating them as efficient and potential targets for treating DNPC.
a Schematic illustration of experimental design and consultant gross photos of major prostate tumors and lung metastases from castrated hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. b Desk summarizing the pathological abnormalities within the prostates of intact and castrated mice. c Consultant photos of H&E and IHC staining utilizing the indicated antibodies on adjoining prostate and lung tissues d Heatmap exhibiting the expression patterns of DEGs from the comparisons of bulk RNA-seq knowledge from prostate tissues from castrated and intact hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice. Purple and blue colours point out up- and down-regulation, respectively. See Supplementary Knowledge 7. e GSEA outcomes from pre-ranked DEG listing evaluating castrated versus intact TripleTg samples. f qPCR evaluation of the indicated genes proven as fold change in prostate tissues from the indicated mice. Knowledge are imply ± s.d. of three organic replicates. **P < 0.01, ***P < 0.001; Unpaired two-tailed t-tests. g Schematic illustration of the experimental design for the ex vivo organoid tradition carried out. Organoids derived from prostate tumor cells of castrated hHGFtg:H11hMET/+:Ctnnb1L(Ex3)/+:PBCre4 mice had been handled with automobile, ENZ, Selinexor, or CX5461 in presence or absence of DHT for six days. See Strategies part. h Consultant photos of brightfield and H&E staining of the organoids with the indicated remedies. i Schematic of hypothetic fashions by which present ADT induces DNPC improvement by HGF/Wnt-induced activation of XPO1 and ribosome biogenesis by SP1. Consultant photos with constant outcomes from three organic replicates are proven. Scale bars, 5 mm (a), 400 μm (h), 100 μm (c), 25 μm (c, h). Supply knowledge and the precise P values are offered within the Supply Knowledge file.