ATP6V0A1 contributes to an immunosuppressive TME by means of exogenous lipids in CRC
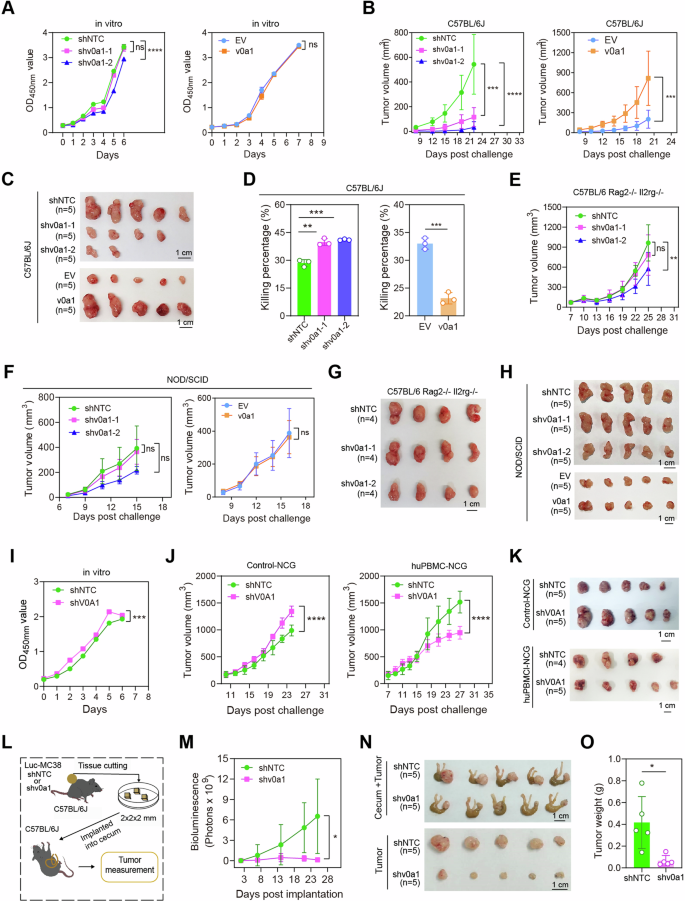
A high-fat food plan (HFD) is understood to induce an immunosuppressive TME in CRC, however the mechanism stays unclear. V-ATPase subunits are important for lipid homeostasis in varied cell varieties5. To analyze the potential roles of V-ATPase subunits in obesity-suppressed anti-tumor immunity in CRC, we stratified 471 TCGA-COAD samples into two clusters with excessive and low lipid metabolism in line with their transcriptomic ranges of lipid metabolism pathways (Supplementary Fig. 1A). The expression ranges of 24 human V-ATPase subunits have been in contrast between these two clusters. As proven in Supplementary Fig. 1B, the expression of 11 V-ATPase subunits, together with ATP6V0A1, ATP6V0C, ATP6V0D1, ATP6V0D2, ATP6V0E1, ATP6V0E2, ATP6V1B1, ATP6V1D, ATP6V1E1, ATP6V1F, and ATP6AP1, have been considerably elevated within the CRCs with excessive lipid metabolism. In parallel, the TCGA-COAD samples have been divided into three teams in line with immune scores (Supplementary Fig. 2A). Among the many 11 V-ATPase subunits correlated with excessive lipid metabolism, ATP6V0A1 confirmed the strongest inverse correlation with immune exercise. This was evident in COAD samples, as ATP6V0A1 was the one subunit that persistently decreased because the immune scores progressively elevated from low to excessive ranges (Supplementary Fig. 2B). Importantly, ATP6V0A1 expression was discovered to be positively related to decreased immune exercise in CRCs with excessive lipid metabolism, whereas no correlation was noticed in CRCs with low lipid metabolism (Supplementary Fig. 3). Then again, excessive lipid metabolism was correlated with decrease immune exercise in CRCs with excessive ATP6V0A1 expression, whereas the immune exercise was comparable between subpopulations with excessive and low lipid metabolism individually in low-ATP6V0A1 CRCs (Supplementary Fig. 4). Elevated lipid metabolism in tumor cells is persistently related to elevated lipid ranges within the TME1. The above information steered that ATP6V0A1 is likely to be important for exogenous lipid-suppressed anti-tumor immunity in CRC. To check this speculation, we investigated the roles of ATP6V0A1 in exogenous lipid-induced CRC immune evasion utilizing an MC38 tumor mannequin in mice with weight problems (Fig. 1A–D). As proven in Fig. 1, HFD promoted the expansion of MC38 tumors (Fig. 1E, F) and suppressed the tumor-killing exercise of their TILs (Fig. 1G, H). Notably, the depletion of ATP6V0A1 considerably attenuated the alterations induced by the HFD (Fig. 1E–H), indicating that ATP6V0A1 performs an important function in regulating CRC immune-evasion by means of the utilization of exogenous lipid within the TME.
A Schematic diagram displaying an animal mannequin to analyze the importance of CRC cell-derived ATP6V0A1 within the suppression of anti-tumor immunity induced by elevated degree of exogenous lipids. B–D C57BL/6 J mice have been fed with high-fat food plan (HFD) or management food plan (CD) for 9 weeks previous to tumor cell implantation, and the physique weight was measured (B); utilizing a body-weight randomization grouping strategy, HFD mice and CD mice have been individually divided into two teams with related physique weights (C) and comparable serum ranges of LDL-cholesterol (D). E–H Management MC38 (shNTC) cells or ATP6v0a1-knockdown (shv0a1) MC38 cells have been subcutaneously injected to HFD mice and CD mice as proven in (A). Tumor volumes have been monitored utilizing calipers, and common tumor development curves have been plotted (E); pictures of the tumors are proven in (F). Tumor-infiltrating lymphocytes (TILs) have been remoted and co-cultured with CFSE-labeled MC38 cells, and the cell combination was analyzed by movement cytometry for the proportion of tumor cell dying to evaluate the killing actions of TILs (G). The relative cytotoxicity of TILs between CD-treated and HFD-treated MC38-shNTC tumors or between CD-treated and HFD-treated MC38-shv0a1 tumors (H) have been assessed by calculating the ratio of tumor cell-death proportion (G) between the cell combination containing TILs from these tumors. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing strange two-way ANOVA (in B, E) or unpaired two-sided Scholar’s t-test (in C, D, G, H). n = 10 (B), 5 (C–F), or 3 (G, H) mice in every group; Information consultant three unbiased experiments (B–H). Supply information and actual p-value are supplied as a Supply Information file.
Tumor cell-intrinsic ATP6V0A1 promotes the expansion of CRC in an immune-dependent method
We initially evaluated the expression degree of ATP6V0A1 in CRC tumor cells. As proven in Supplementary Fig. 5A, B, ATP6V0A1 protein ranges have been considerably increased in 6 human CRC cell strains (CACO2, HCT-8, HCT-116, HT-29, RKO, and SW620) than in regular human colon epithelial NCM460 cells. ATP6V0A1 was additionally overexpressed in 2 murine CRC cell strains (MC38 and CT26) in contrast with regular murine colon (Supplementary Fig. C). We then stably knocked down Atp6v0a1/ATP6V0A1 in mouse (MC38 and CT26) and human (HCT8) CRC cell strains, and in addition created an Atp6v0a1-overexpressing clone in MC38 cells (Supplementary Fig. 5D, E). Subsequently, we employed these modified cells to analyze the influence of tumor cell-intrinsic ATP6V0A1 on suppressing anti-tumor immunity in syngeneic and xenograft mouse fashions of CRC (Supplementary Fig. 6).
CCK8 assays revealed that the expansion of MC38 cells in vitro was not affected by both Atp6v0a1 knockdown or Atp6v0a1 overexpression (Fig. 2A). Apparently, Atp6v0a1 knockdown in MC38 cells considerably suppressed tumor development (Fig. 2B, C) and restored the tumor-killing exercise of TILs (Fig. 2D) in immunocompetent C57BL/6 J mice, whereas Atp6v0a1 overexpression had the alternative impact (Fig. 2B–D). The alterations in tumor development attributable to both ATP6V0A1 depletion or overexpression have been fully abolished or considerably lowered in immunodeficient Rag2−/−Il2rg−/− or NOD/SCID mice (Fig. 2E–H). These information demonstrated that ATP6V0A1 enhances MC38 tumor development by inhibiting the anti-tumor immune response. Much like the outcomes noticed in MC38 cells, knockdown of Atp6v0a1 in CT26 cells didn’t suppress their development charge in vitro (Supplementary Fig. 7A). Furthermore, depletion of ATP6V0A1 nearly fully inhibited CT26 tumor development in immunocompetent BALB/c mice, whereas solely barely retarded tumor development in immunodeficient BRG or NOD/SCID mice (Supplementary Fig. 7B–D). Notably, on the respective termination time factors, tumor weights have been comparable for management CT26 tumors in BALB/c mice and immunodeficient mice however considerably decrease for CT26-shv0a1 tumors in BALB/c mice than in immunodeficient mice, displaying that Atp6v0a1 interference displays better efficacy in suppressing the expansion of CT26 tumors in immunocompetent mice than in immunodeficient mice (Supplementary Fig. 7B–D). These findings steered that immune responses are important for regulating CT26 tumor development by ATP6V0A1, though different elements may be concerned.
A The impact of suppressing or overexpressing Atp6v0a1 on the proliferation charges of MC38 cells in vitro was analyzed by CCK8 assay. n = 3 unbiased experiments. B–D C57BL/6 J mice have been subcutaneously injected with Atp6v0a1-suppressing or Atp6v0a1-overexpressing MC38 cells as proven in Supplementary Fig. 6A. Tumor volumes have been monitored utilizing calipers, and common tumor development curves have been plotted (B); pictures of the tumors are proven in (C). n = 5 mice per group. TILs remoted from the tumors described in (B) and (C) have been co-cultured with CFSE-labeled MC38 cells, and the cell combination was analyzed by movement cytometry for the proportion of tumor cell dying to evaluate the killing actions of TILs (D). n = 3 mice in every group; Information consultant three unbiased experiments. E–H Atp6v0a1-suppressing or Atp6v0a1-overexpressing MC38 cells have been subcutaneously injected into C57BL/6 Rag2−/−Il2rg−/− mice (E, G) or NOD/SCID mice (F, H) as proven in supplementary Fig. 6A. Common tumor development curves have been plotted (E, F); pictures of the tumors are proven in (G, H). n = 4 (E, G) or 5 (F, H) mice per group. I The proliferation of management HCT-8 (shNTC) cells and ATP6V0A1-knockdown (shV0A1) HCT-8 cells in vitro was analyzed by CCK8 assay. n = 3 unbiased experiments. J, Okay HCT-8 shNTC cells or HCT-8 shV0A1 cells have been subcutaneously injected into huPBMC-NCG mice and management NCG mice as proven in Supplementary Fig. 6B. Common tumor development curves have been plotted (J); pictures of the tumors are proven in (Okay). n = 4 (shNTC group in huPBMC-NCG mice mannequin) or 5 mice (different teams) in every group. L–O Cecal MC38 tumors have been established as illustrated in (L). Tumor development was monitored by way of bioluminescence detection, and common tumor development curves have been plotted (n = 5 mice per group; M). The mice have been sacrificed on day 25; pictures of the tumors within the cecum (N, higher) and the remoted tumors (N, decrease) are proven. Tumor weights for the indicated teams are in contrast in (O). For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing strange two-way ANOVA (A, B, E, F, I, J, M) or unpaired two-sided Scholar’s t-test (D, O). Supply information and actual p-value are supplied as a Supply Information file.
To analyze the function of ATP6V0A1 in anti-tumor immunity within the context of human immune and colon most cancers cells, we established a subcutaneous human colon most cancers mannequin in immunodeficient mice engrafted with human-derived immune cells (Supplementary Fig. 6B, C). Evaluation of cell proliferation/tumor development confirmed that ATP6V0A1 knockdown in human HCT8 colon most cancers cells barely (however considerably) elevated their proliferation in vitro (Fig. 2I) and in immune-deficient NCG mice (Fig. 2J, Okay) however considerably suppressed their development in immune-reconstituted huPBMC-NCG mice (Fig. 2J, Okay). These outcomes steered that ATP6V0A1 additionally promoted immune evasion in human colon cancers. Subsequent, to check whether or not tumor cell-intrinsic ATP6V0A1 may promote the event of orthotopic CRCs, we established a cecal MC38 tumor mannequin (Fig. 2L). Much like the subcutaneous tumor mannequin, knockdown of Atp6v0a1 in MC38 cells additionally considerably suppressed in vivo tumor development within the cecal mannequin (Fig. 2M–O). Collectively, these outcomes point out that tumor cell-intrinsic ATP6V0A1 suppresses anti-tumor immune responses and thus promotes tumor development throughout completely different colon most cancers fashions.
Tumor cell-intrinsic ATP6V0A1 suppresses reminiscence CD8+ T cells exercise in CRC
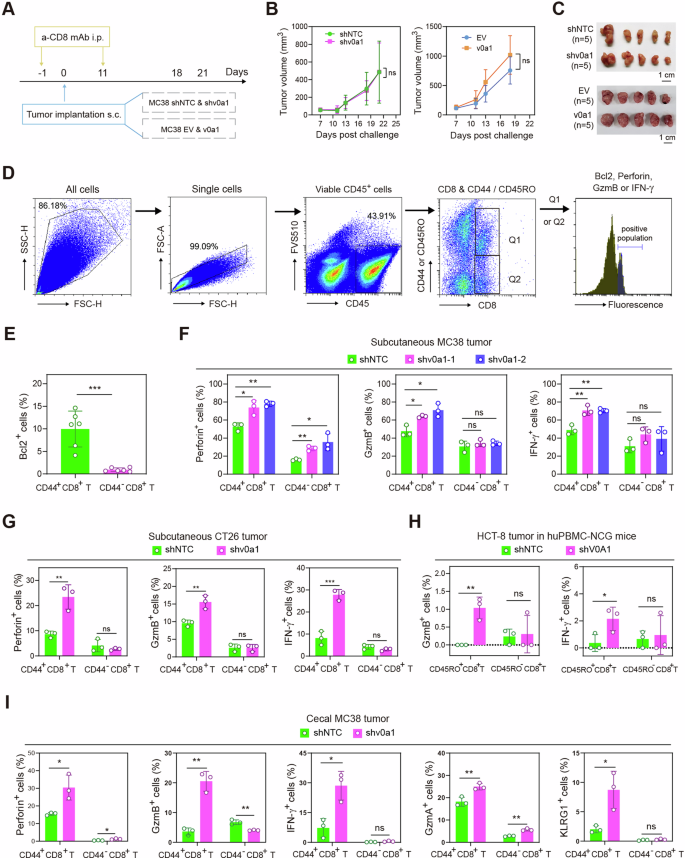
To find out the kind of immune cells contributing to ATP6V0A1-mediated immune evasion, we used single-cell transcriptome sequencing (scRNA-seq) to investigate the ATP6V0A1-edited immune microenvironment in CRC. Holistic immune profiles in Atp6v0a1 knockdown (shv0a1, n = 5) and management MC38 tumors (shNTC, n = 5) have been in contrast (Supplementary Figs. 8, 9) as a technique described in strategies. The outcomes confirmed that Atp6v0a1 interference in MC38 cells elevated the odds of naïve T, effector T, and memory-like T-2 cells within the complete T cell inhabitants whereas reducing the odds of exhausted T, Treg, and memory-like T-1 cells (Supplementary Fig. 9A, B). Furthermore, the odds of granulocytes, monocytes, and dendritic cells throughout the myeloid inhabitants have been elevated in Atp6v0a1 knockdown-derived tumors, whereas the proportion of macrophages was decreased (Supplementary Fig. 9C, D). Apparently, memory-like T-2 cells have been detected in shv0a1 however not shNTC MC38 tumors (Supplementary Fig. 9A, B). Of all of the immune cell subclusters, memory-like T-2 cells exhibited essentially the most considerably elevated cell rely ratio in shv0a1 tumors vs. management tumors (Supplementary Fig. 9E). As well as, memory-like T-2 cells expressed extra effector markers than memory-like T-1 cells (Supplementary Fig. 8D). According to these findings, the expression ranges of TCR signaling molecules and T-cell activation markers in complete memory-like T cells have been increased in shv0a1 tumors than in shNTC tumors (Supplementary Fig. 9F). These information recommend that MC38-derived ATP6V0A1 primarily regulates anti-tumor immunity by suppressing the effectiveness of reminiscence T cells throughout the CRC TME.
Reminiscence-like T-2 cells preferentially specific CD8 moderately than CD4 (Supplementary Fig. 8D). Due to this fact, we initially examined the function of CD8+ T cells in ATP6V0A1-regulated immune evasion within the MC38 allograft mannequin (Fig. 3A). Depletion of CD8+ T cells by way of administration of monoclonal anti-CD8α (Clone 2.43) overcame the suppression/acceleration of MC38 tumor development induced by the knock-down/over-expression of MC38-derived Atp6v0a1 (Fig. 3B, C). This discovering steered that MC38-derived ATP6V0A1 promoted tumor immune evasion primarily by suppressing CD8+ T-cell exercise. Subsequent, we used a movement cytometry (FC) technique (Fig. 3D) to verify whether or not ATP6V0A1 selectively affected the exercise of reminiscence CD8+ T cells in murine colon cancers. For these assays, we used CD44 as a protein marker of murine reminiscence CD8+ T cells14. FC evaluation additionally confirmed that BCL-2, one other particular marker of memory-like T cells recognized within the scRNA-seq evaluation (Supplementary Fig. 8D), was most continuously detected in CD44+CD8+ moderately than CD44−CD8+ T cells (Fig. 3E); this discovering indicated that CD44+CD8+ T cells might signify the T-cell populations annotated as memory-like T cells in our scRNA-seq evaluation. FC evaluation of effector cytokines confirmed that knockdown of Atp6v0a1 in each MC38 and CT26 cells considerably enhanced the degrees of perforin, GzmB, and IFN-γ in CD44+CD8+ T cells derived from the corresponding subcutaneous tumors (Fig. 3F, G). Against this, the degrees of those cytokines in CD44−CD8+ T cells weren’t persistently enhanced in MC38 tumors and CT26 tumors with Atp6v0a1 knockdown (Fig. 3F, G). According to these findings, ATP6V0A1 knockdown in HCT8 cells considerably enhanced the effectiveness of reminiscence CD45RO+CD8+ T cells however not that of CD45RO−CD8+ T cells in hPBMC-NCG mouse-derived HCT8 tumors (Fig. 3H). Importantly, the degrees of cytotoxic cytokines in CD44+CD8+ T cells have been additionally considerably enhanced within the orthotopic (cecal) MC38 tumor mannequin on account of Atp6v0a1 knockdown (Fig. 3I). Much like the subcutaneous MC38 tumor mannequin, knockdown of tumor cell-intrinsic Atp6v0a1 didn’t persistently improve the extent of the cytotoxic cytokines in CD44−CD8+ T cells derived from the cecal tumor mannequin (Fig. 3I).
A–C CD8+ T cells have been depleted from C57BL/6 mice within the indicated teams by injection of anti-CD8α mAb (clone 2.43) on the time factors proven (A). Common tumor development curves have been plotted (B); pictures of the tumors are proven in (C). n = 5 mice per group. D Movement cytometry (FC) technique for gating CD44+CD8+ and CD44–CD8+ T cells and detecting effector cytokines. E BCL2 protein ranges have been in contrast in CD44+CD8+ and CD44−CD8+ T cells by FC. n = 6; Information are pooled from 2 unbiased experiments. F–I The effectiveness of tumor-infiltrating CD44+CD8+ and CD44−CD8+ T cells or CD45RO+CD8+ and CD45RO−CD8+ T cells was analyzed by way of FC in subcutaneous MC38 tumors from C57BL/6 J mice (F), subcutaneous CT26 tumors from BALB/c mice (G), subcutaneous HCT-8 tumors from huPBMC-NCG mice (H), and cecal MC38 tumors from C57BL/6 J mice (I). n = 3 mice per group; Information consultant of three unbiased experiments. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001. Statistical significance was decided utilizing strange two-way ANOVA (B) or unpaired two-sided Scholar’s t-test (E–I). Supply information and actual p-value are supplied as a Supply Information file.
Collectively, these information recommend that ATP6V0A1 expressed in CRC tumor cells promotes immune evasion primarily by suppressing the effectiveness of reminiscence CD8+ T cells.
TGF-β1/SMAD3 paracrine axis hyperlinks tumor cell-intrinsic ATP6V0A1 to the suppression of reminiscence CD8+ T cells exercise
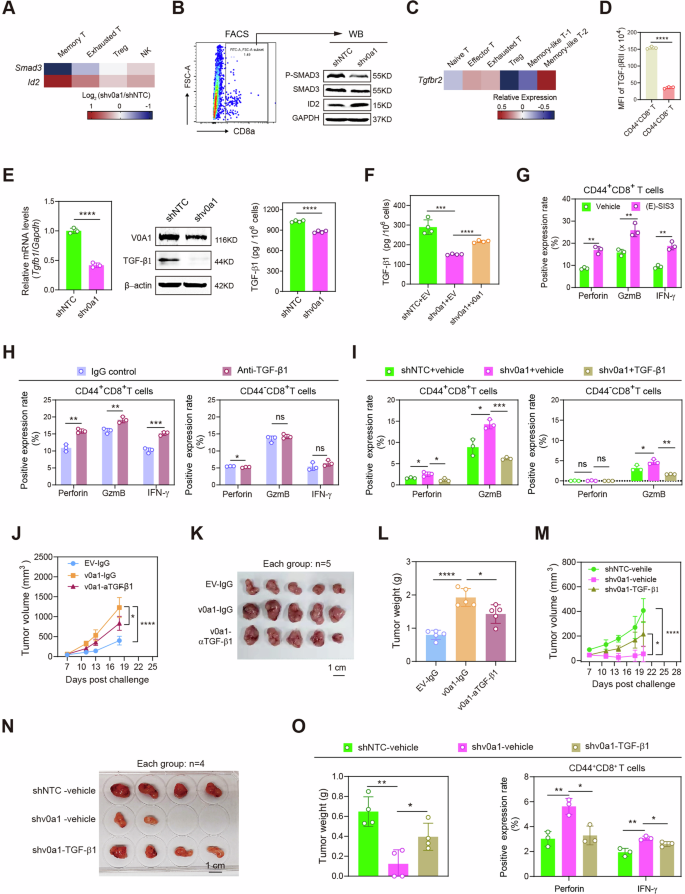
To discover the molecular mechanisms underlying the cross-talk between ATP6V0A1 and reminiscence CD8+T cells, we first used the scRNA-seq information to establish genes that have been differentially expressed in memory-like T cells from MC38-shNTC and MC38-shv0a1 tumors. Transcriptomic comparability confirmed that following Atp6v0a1 interference in MC38 cells, the extent of Smad3 mRNA was considerably lowered and the extent of Id2 mRNA was enhanced in memory-like T cells, relative to the degrees in different T-cell subpopulations (Fig. 4A). Id2 has been reported to be repressed by SMAD3 signaling15. Furthermore, western blotting confirmed decreases in complete SMAD3 and phosphorylated SMAD3 (p-SMAD3) protein ranges and a rise in ID2 protein ranges in CD8+ T cells from MC38-shv0a1 tumors, in contrast with ranges in MC38-shNTC tumors (Fig. 4B and Supplementary Fig. 10A). SMAD3 signaling is usually activated by the TGF-β/TGF-βR axis16. Apparently, Tgfbr2 mRNA was extra extremely expressed in memory-like T-2 cells than in different T cell subclusters (Fig. 4C). According to this discovering, TGF-βRII protein ranges in wild-type MC38 tumors have been increased in CD44+CD8+ T cells than in CD44−CD8+ T cells (Fig. 4D). In the meantime, Tgfb1 mRNA ranges and TGF-β1 protein ranges have been considerably decrease in Atp6v0a1-suppressing MC38 cells than in management cells (Fig. 4E and Supplementary Fig. 10B); Overexpression of Atp6v0a1 can restore the decreased TGF-β1 ranges induced by Atp6v0a1 knockdown (Fig. 4F). IHC evaluation confirmed that intra-tumoral TGF-β1 ranges have been decrease in MC38-shv0a1 tumors than in MC38-shNTC tumors (Supplementary Fig. 10C, D). The above information indicated that tumor-intrinsic ATP6V0A1 might regulate reminiscence CD8+ T cells by way of paracrine TGF-β1/SMAD3 signaling. Importantly, therapy of MC38 tumor-derived TILs with the SMAD3 inhibitor (E)-SIS3 considerably enhanced the expression of effector cytokines in CD44+CD8+ T cells (Supplementary Fig. 11 and Fig. 4G), confirming the important function of SMAD3 activation in suppressing reminiscence CD8+ T cell operate. Moreover, pretreatment of MC38 cell tradition medium with anti-TGF-β1 resulted in considerably elevated expression of effector cytokines in CD44+CD8+ T cells however not in CD44−CD8+ T cells within the in vitro tradition of CD8+ T cells with MC38 cell tradition medium (Fig. 4H). The expression of effector cytokines was additionally considerably increased in CD44+CD8+ T cells handled with conditioned medium (CM) from Atp6v0a1 knockdown MC38 cells however not in these handled with CM from management MC38 cells, whereas pretreatment of the CM with recombinant mouse TGF-β1 eradicated this enhancement (Fig. 4I). These information indicated the necessary function of TGF-β1/SMAD3 signaling within the suppression of reminiscence CD8+ T cell operate by MC38-derived Atp6v0a1. We went on to additional verify the contribution of TGF-β1 to Atp6v0a1-mediated regulation of tumor immune evasion in vivo. Experiments performed within the syngeneic mouse tumor mannequin confirmed that anti-TGF-β1 therapy suppressed the enhancement of MC38-tumor development promoted by Atp6v0a1 overexpression (Fig. 4J–L). Against this, injection of recombinant mouse TGF-β1 protein into MC38 tumors rescued the expansion suppression attributable to Atp6v0a1 knockdown (Fig. 4M–O). Importantly, TGF-β1 supplementation additionally inhibited the enhancement of CD44+CD8+ T cell exercise mediated by Atp6v0a1 knockdown (Fig. 4O). Furthermore, the restoration of Tgfb1 expression in MC38 tumor cells (Supplementary Fig. 12A) had a comparable influence to exogenous TGF-β1 in mitigating the tumor suppression (Supplementary Fig. 12B–D) and CD44+CD8+ T cell activation (Supplementary Fig. 12E) induced by Atp6v0a1 depletion. It’s of be aware that the suppression of Tgfb1/TGFB1 in MC38 /HCT-8 cells didn’t considerably have an effect on the expansion of those cells (Supplementary Fig. 12F, G). Collectively, our information recommend that the TGF-β1/SMAD3 axis is important for the regulation of the anti-tumor actions of CD44+CD8+ T cells by CRC cell-expressed ATP6V0A1.
A Atp6v0a1 knockdown-induced expression modifications in Smad3 and Id2 have been analyzed within the indicated T-cell subpopulations primarily based on the scRNA-seq information (Supplementary Fig. 8). B Western blotting detecting the indicated protein ranges in CD8+ T cells remoted from MC38-shNTC and MC38-shv0a1 tumors. The samples derive from the identical experiment however completely different gels for SMAD3, ID2, GAPDH, one other for p-SMAD3 have been processed in parallel. C scRNA-seq information evaluating the expression of Tgfbr2 in numerous T-cell subpopulations. D FC-analysis evaluating TGF-βRII degree between CD44+CD8+ and CD44–CD8+ T cells in wild-type MC38 tumors. E, F The mRNA and protein ranges of TGF-β1 in MC38 cells have been analyzed by qPCR (E, left), Western blotting (E, medium), and ELISA (E, proper and F). G–I Wild-type MC38 tumor-derived CD8+ T cells have been handled with the next: (E)-SIS3 (G), MC38 cell tradition medium plus management IgG or anti-TGF-β1 (H), conditioned medium from MC38-shNTC cells or MC38-shv0a1 cells with/with out complement of TGF-β1 protein (I). The activation of CD44+ CD8+ and CD44–CD8+ T cells was decided by FC evaluation. J–L MC38-EV, or MC38-v0a1 cells have been subcutaneously injected into C57BL/6 J mice, adopted by intraperitoneal injection of management IgG or anti-TGF-β1 on days 7, 10, and 13. Common tumor development curves (J), pictures of the tumors (Okay), and a comparability of tumor weights on day 18 (L) are proven; n = 5 mice per group. M–O MC38-shNTC or MC38-shv0a1 cells have been subcutaneously injected into C57BL/6 J mice; PBS or mouse TGF-β1 protein was injected intratumorally. Common tumor development curves (M), pictures of the tumors (N), and a comparability of tumor weights on day 20 (O, left) are proven. TILs from day 20 tumors have been analyzed for effector manufacturing in CD44+CD8+ T cells (O, proper). n = 4 mice per group. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing strange two-way ANOVA (in J, M) or unpaired two-sided Scholar’s t-test (in D, E, F, G, H, I, L, O). n = 3 unbiased experiments for Fig. 4D–I; Three unbiased experiments have been carried out for Fig. 4B, E (medium). Supply information and actual p-value are supplied as a Supply Information file.
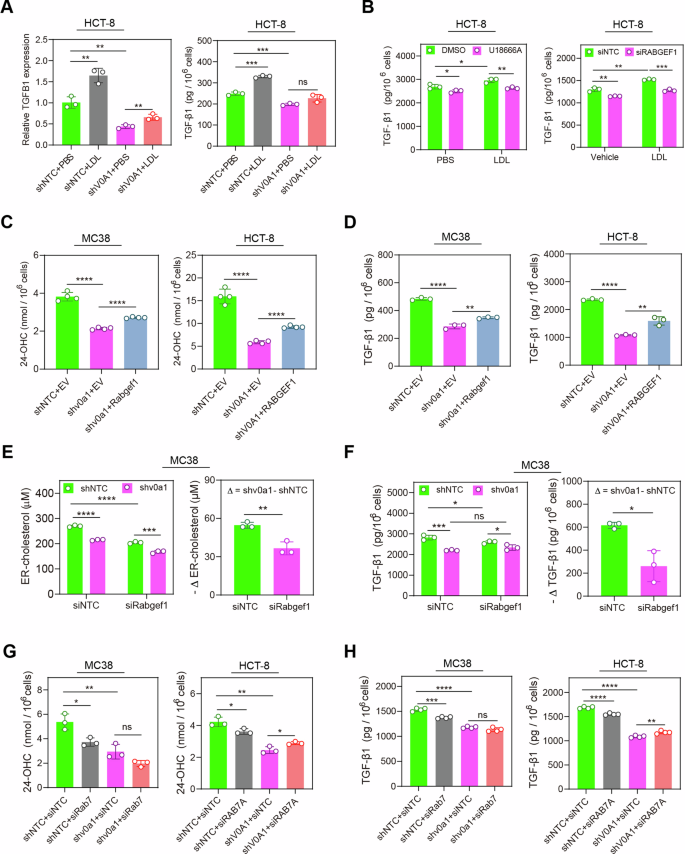
Enhanced degree of ldl cholesterol and its 24-OHC manufacturing within the ER mediates ATP6V0A1-induced upregulation of TGF-β1
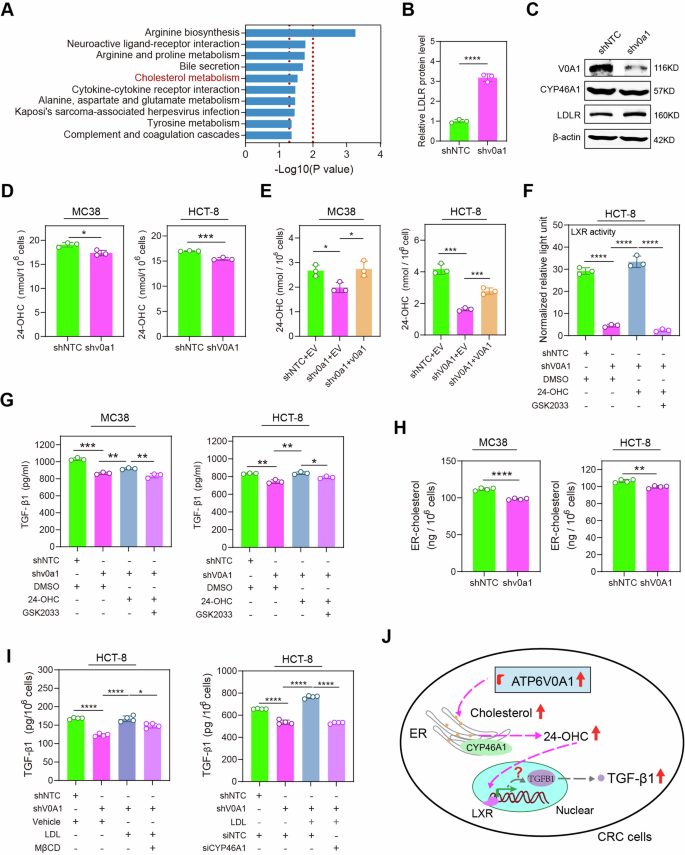
To discover the molecular mechanisms underlying ATP6V0A-mediated TGF-β1 upregulation in CRC cells, we utilized a quantitative proteomics strategy to analyze the proteins related to ATP6V0A1. As beforehand talked about, lipid metabolic reprogramming is crucial for the inhibition of anti-tumor immunity by means of ATP6V0A1. Notably, a key molecular cluster associated to ldl cholesterol metabolism signaling was considerably altered in MC38-shv0a1 cells in comparison with the management MC38 cells (Fig. 5A). As well as, LDLR was upregulated significantly in MC38-shv0a1 cells (Fig. 5B, C; Supplementary Fig. 13). Ldl cholesterol metabolism is an important facet of lipid metabolism related to regulating TGF-β1 expression17. The mechanism underlying the expression of TGF-β1 induced by ldl cholesterol metabolism stays unclear. Due to this fact, we aimed to analyze whether or not ATP6V0A1 may management the expression of TGF-β1 by means of reprogramming ldl cholesterol metabolism. LDLR upregulation is usually recommended to point a lower within the ranges of oxysterols, notably 24-OHC, produced within the ER18,19. Certainly, Atp6v0a1/ATP6V0A1 knockdown in MC38/HCT-8 cells considerably lowered the manufacturing of 24-OHC in cell tradition (Fig. 5D). Restoration of Atp6v0a1/ATP6V0A1 expression in ATP6V0A1-deficient MC38/HCT-8 cells have the potential to reverse the decreased ranges of 24-OHC (Fig. 5E). 24-OHC is a typical LXR agonist that exerts its organic capabilities by activating LXR signaling19. Persistently, ATP6V0A1 knockdown in HCT-8 cells lowered LXR exercise as demonstrated by the LXR luciferase reporter assays, whereas 24-OHC therapy may fully rescue this decreased LXR exercise (Fig. 5F). Furthermore, therapy with GSK2033, an LXR inhibitor, suppressed the results of 24-OHC on inducing LXR activation (Fig. 5F). These information steered that ATP6V0A1 drives the activation of 24-OHC/LXR pathway. We additional explored the roles of 24-OHC/LXR pathway in ATP6V0A1-induced TGF-β1 expression. Exogenous therapy with 24-OHC partially reversed the suppression of TGF-β1 ranges in Atp6v0a1-suppressing MC38 cells, whereas fully reversing it in ATP6V0A1-suppressing HCT-8 cells (Fig. 5G). Moreover, therapy with GSK2033 reversed the influence of 24-OHC on TGF-β1 ranges in ATP6V0A1 knockdown cells (Fig. 5G). Equally, downregulation of Nr1h3 (LXRα) or Nr1h2 (LXRβ) additionally inhibited the results of 24-OHC on rising TGF-β1 ranges. Combining deficiencies in LXRα and LXRβ yielded more practical ends in counteracting the results of 24-OHC in comparison with having solely one of many deficiencies (Supplementary Fig. 14A), suggesting that each LXRα and LXRβ are concerned within the technique of ATP6V0A1 inducing TGF-β1 expression by way of 24-OHC manufacturing. These outcomes confirmed that ATP6V0A1 promotes TGF-β1 expression by way of the 24-OHC/LXR pathway in CRC cells. 24-OHC is produced within the ER by the oxidation of ldl cholesterol, catalyzed by CYP46A119. Nevertheless, the degrees of CYP46A1 weren’t considerably altered by Atp6v0a1 knockdown (Fig. 5C; Supplementary Fig. 13). Apparently, the degrees of ER-derived ldl cholesterol have been decreased in each Atp6v0a1-suppressing MC38 cells and ATP6V0A1-suppressing HCT-8 cells, relative to the degrees in management cells (Fig. 5H). Furthermore, treating HCT-8 cells with LDL to complement ldl cholesterol within the ER restored the decreased 24-OHC manufacturing (Supplementary Fig. 14B) and lowered TGF-β1 expression (Fig. 5I) attributable to ATP6V0A1 knockdown. Remedy with MβCD to lower mobile ldl cholesterol can reverse the results induced by LDL (Supplementary Fig. 14B; Fig. 5I). Importantly, suppressing 24-OHC manufacturing by way of CYP46A1 knockdown (Supplementary Fig. 14B) successfully counteract the TGF-β1 expression elevated by exogenous LDL therapy (Fig. 5I). Collectively, these information demonstrated that ATP6V0A1 promotes 24-OHC manufacturing primarily by enhancing levels of cholesterol within the ER, thereby rising TGF-β1 expression by way of the LXR pathway (Fig. 5J).
A, B Atp6v0a1 knockdown-induced modifications in protein ranges in MC38 cells have been analyzed utilizing label-free protein quantitative mass spectrometry (MS); KEGG route enrichment evaluation confirmed that modifications within the ldl cholesterol metabolism pathway have been induced by Atp6v0a1 knockdown (A). Relative ranges of LDLR protein in MC38-shNTC and MC38-shv0a1 cells have been decided by MS evaluation (B; n = 3 unbiased experiments). C Western blotting was used to detect LDLR and CYP46A protein ranges in MC38-shNTC and MC38-shv0a1 cells. The samples derive from the identical experiment however completely different gels for ATP6V0A1, CYP46A1, β-actin, one other for LDLR have been processed in parallel. Information consultant of three unbiased experiments. D, E Tradition media from the indicated cells have been analyzed for 24-OHC manufacturing by ELISA. n = 3 unbiased experiments. F HCT-8 (shNTC and shV0A1) cells with the indicated remedies have been transfected with LXR luciferase reporter plasmids together with renilla luciferase management plasmids. The cells have been subsequently assessed for LXR actions by calculating the ratio of luciferin gentle unit to renilla gentle unit (normalized relative gentle unit). n = 3 unbiased experiments. G MC38 (shNTC and shv0a1) cells and HCT-8 (shNTC and shV0A1) cells have been handled with 24-OHC within the absence or presence of LXR inhibitor (GSK2033), and TGF-β1 ranges within the supernatants have been analyzed by ELISA. n = 3 unbiased experiments. H Following the isolation of ER from the indicated cells, lipids have been extracted and analyzed for levels of cholesterol utilizing the Amplex™ Pink ldl cholesterol assay. n = 4 unbiased experiments. I HCT-8 (shNTC and shV0A1) cells have been handled with LDL within the absence or presence of MβCD (I, left) or with LDL within the presence of siNTC or siCYP46A1 (I, proper); TGF-β1 protein was detected within the supernatant by ELISA. n = 4 unbiased experiments. J Schematic diagram summarizing the outcomes of Fig. 5A–I. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing unpaired two-sided Scholar’s t-test. Supply information and actual p-value are supplied as a Supply Information file.
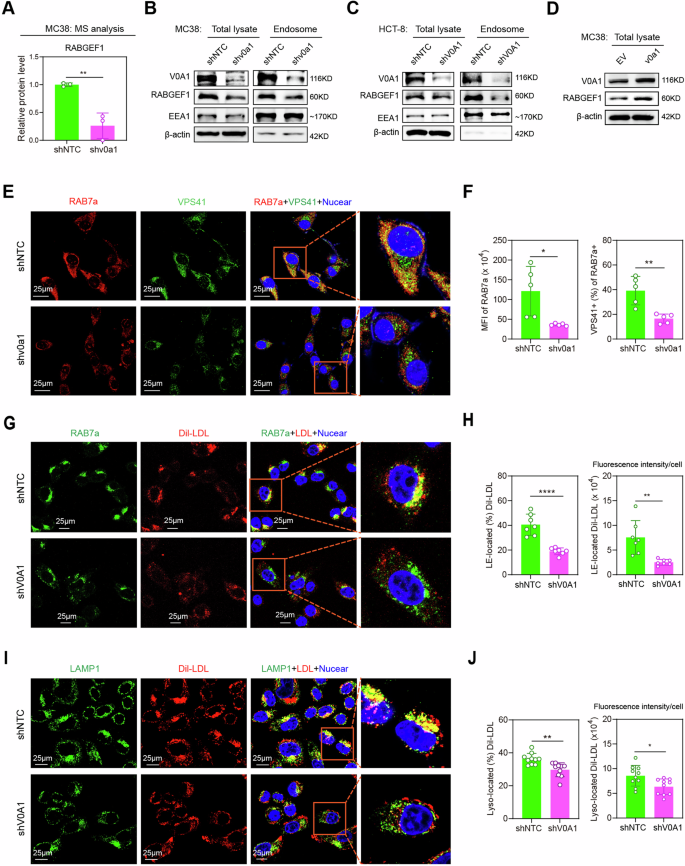
ATP6V0A1 facilitates the transportation of exogenous ldl cholesterol to the ER by way of the RABGEF1-dependent endosome maturation pathway
Having proven that ATP6V0A1 mediated the upregulation of TGF-β1 expression by reprogramming ldl cholesterol metabolism in CRC cells, we needed to analyze the mechanism by which ATP6V0A1 enhanced levels of cholesterol within the ER. First, we confirmed that Atp6v0a1 knockdown in MC38 cells didn’t considerably alter the extent of HMGCR, the important thing rate-limiting enzyme for endogenous ldl cholesterol synthesis (Supplementary Fig. 15). In the meantime, quantitative proteomics and western blotting evaluation revealed that the RABGEF1 endosome maturation pathway, which is important for vesicular transport20, was suppressed by Atp6v0a1/ATP6V0A1 knockdown in MC38 cells and HCT-8 cells (Fig. 6A–C; Supplementary Fig. 16A, B). Then again, the RABGEF1 degree was elevated by Atp6v0a1 overexpression in MC38 cells (Fig. 6D and Supplementary Fig. 16C). Importantly, confocal microscopy confirmed that Atp6v0a1 knockdown in MC38 cells resulted in considerably decreased RAB7a expression in endosomes and decreased VPS41 ranges in RAB7a+ endosomes (Fig. 6E, F). Furthermore, the lowered ranges of endosomal RAB7a in Atp6v0a1 knockdown cells have been restored following Rabgef1 overexpression in MC38 cells (Supplementary Fig. 17A, B). RAB7a and VPS41 are the markers of late endosomes, and are required for endosomes to mature into degradative lysosomal compartments20. These information demonstrated that ATP6V0A1 helps RABGEF1-dependent endosome maturation. Due to this fact, ATP6V0A1 might improve ER levels of cholesterol by way of the regulation of ldl cholesterol transport. In addition to being lowered within the ER (Fig. 5H), levels of cholesterol have been additionally decreased in endosomes and lysosomes in Atp6v0a1-suppressing MC38 cells and ATP6V0A1-suppressing HCT-8 cells (Supplementary Fig. 17C, D), indicating that ATP6V0A1 might assist the absorption of exogenous ldl cholesterol and thus their coming into into ER. To discover this risk, we handled HCT-8-shNTC and HCT-8 shV0A1 cells with exogenous Dil-LDL. Apparently, ATP6V0A1 knockdown lowered the degrees of exogenous Dil-LDL coming into each RAB7a+ late endosomes (Fig. 6G, H) and LAMP1+ lysosomes (Fig. 6I, J) in HCT-8 cells and enhanced the localization of Dil-LDL inside RAB35+ recycling endosomes (Supplementary Fig. 17E, F). According to these findings, in HCT-8 cells handled with Dil-LDL for various lengths of time, ATP6V0A1 knockdown-induced reductions in intracellular Dil-LDL ranges have been detected at later time factors (6 h and eight h), however not at an earlier timepoint (4 h; Supplementary Fig. 18A, B). Furthermore, the extent of recycling of Dil-LDL to the tradition medium in HCT-8 cells was elevated by ATP6V0A1 knockdown (Supplementary Fig. 18C, D). These information demonstrated that ATP6V0A1 facilitates the following transportation of exogenous LDL to the lysosomal degradation pathway, which permits the discharge of LDL-cholesterol after exogenous LDL enters CRC cells. The lysosomal degradation of LDL proteins is required for the transport of exogenous LDL-cholesterol to the ER19. Due to this fact, ATP6V0A1 can facilitates the rise of levels of cholesterol in ER by enhancing the absorption of exogenous ldl cholesterol.
A Atp6v0a1 knockdown-induced modifications within the ranges of RABGEF1 protein have been analyzed in MC38 cells by quantitative mass spectrometry (MS). n = 3 unbiased experiments. B–D Western blotting was used to detect ranges of RABGEF1 protein in complete cells (complete lysate) and endosomes. In (B, C), the samples derive from the identical experiment however completely different gels for RABGEF1, EEA1, β-actin, one other for ATP6V0A1 have been processed in parallel. E, F RAB7a (purple) and VPS41 (inexperienced) proteins in MC38-shNTC and MC38-shv0a1 cells have been detected utilizing confocal fluorescence microscopy (E); quantitative evaluation of vesicle-derived RAB7a ranges (F, left; n = 5 fields per group) and the odds of RAB7a+ vesicles that have been VPS41+ (F, proper; n = 5 fields per group) was carried out utilizing Picture J. G–J HCT-8-shNTC and HCT-8-shV0A1 cells have been handled with 50 µg/ml of human Dil-LDL for six h, and confocal fluorescence microscopy was used to investigate the colocalization of Dil-LDL with RAB7a+ late endosomes (LE; G, H) or LAMP1+ lysosomes (Lyso; I, J). Consultant pictures are proven in (G, I). Quantitative analyses of Dil-LDL localization in endosomes (H; n = 7 fields per group) and lysosomes (J; n = 10 fields per group) have been carried out by measuring the ratio (H, J, left) or the fluorescence depth (H, J, proper) of Dil-LDL situated in these vesicles with Picture J. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ****p < 0.0001. Statistical significance was decided utilizing unpaired two-sided Scholar’s t-test. MFI imply fluorescence depth = fluorescence depth/cell. Three unbiased experiments have been carried out for Fig. 6B–J. Supply information and actual p-value are supplied as a Supply Information file.
We additional explored the function of ATP6V0A1 in exogenous cholesterol-induced TGF-β1 expression. The depletion of ATP6V0A1 considerably counteracted the elevated TGF-β1 degree by LDL therapy (Fig. 7A), illustrating the need of ATP6V0A1 for exogenous ldl cholesterol to upregulate TGF-β1 expression. RABGEF1-dependent endosome maturation is essential for ldl cholesterol absorption, because the inhibition of endosome maturation by means of RABGEF1 knockdown hindered the transportation of exogenous LDL to late endosomes (Supplementary Fig. 19A, B). Importantly, inhibition of ldl cholesterol absorption by way of therapy with U18666A, an inhibitor of ldl cholesterol transport from lysosome to ER, or by way of RABGEF1 knockdown attenuated the enhancement of TGF-β1 ranges induced by exogenous LDL in HCT-8 cells (Fig. 7B). These information indicated that RABGEF1-dependent ldl cholesterol absorption is important for exogenous ldl cholesterol to induce TGF-β1 expression. Furthermore, related with ATP6V0A1 suppression, blockade of ldl cholesterol absorption lowered TGF-β1 expression primarily by reducing 24-OHC manufacturing (Supplementary Fig. 19C, D). We thus discover whether or not ATP6V0A1 regulates 24-OHC-mediated TGF-β1 expression by way of RABGEF1-dependent ldl cholesterol absorption. Overexpression of Rabgef1 in ATP6V0A1-suppressing MC38 or CT26 cells or overexpression of RABGEF1 in ATP6V0A1-suppressing HCT-8 cells may restore the 24-OHC manufacturing and TGF-β1 expression lowered by Atp6v0a1/ATP6V0A1 knockdown (Fig. 7C, D; Supplementary Fig. 20). Persistently, inhibiting ldl cholesterol absorption with siRabgef1 mitigated the discount in ER-cholesterol (Fig. 7E) and TGF-β1 (Fig. 7F) in Atp6v0a1-suppressing MC38 cells. Endosome maturation and acidification are required for RABGEF1-dependent ldl cholesterol absorption. Consequently, we explored the capabilities of RAB7a, a key issue of endosome maturation, in ATP6V0A1-regulated TGF-β1 expression. Knockdown of Rab7 or RAB7A in MC38 or HCT-8 cells counteracted the lower in each 24-OHC (Fig. 7G) and TGF-β1 (Fig. 7H) ranges induced by ATP6V0A1 suppression, with a extra pronounced impact in MC38 cells. Endosome acidification outcomes from endosome maturation and additional drives the endo-lysosomal site visitors for ldl cholesterol absorption. As anticipated, ATP6V0A1 suppression didn’t alter the mobile pH (Supplementary Fig. 21A) however enhanced the pH in RAB7a+ endosomes (Supplementary Fig. 21B, C). It’s of be aware that ATP6V0A1 most popular to control the pH of RAB7a+ vesicles moderately than RAB7a- vesicles (Supplementary Fig. 21B, D). Importantly, the blockade of endosome acidification with Baf-A1 therapy eradicated the lower in each 24-OHC and TGF-β1 ranges induced by ATP6V0A1 suppression in HCT-8 cells (Supplementary Fig. 21E). Collectively, these information steered that ATP6V0A1 facilitates 24-OHC-mediated TGF-β1 expression by means of the ldl cholesterol absorption pushed by RABGEF1-dependent endosome maturation.
A HCT-8-shNTC and HCT-8-shV0A1cells have been handled with 20 µg/ml of human Dil-LDL within the tradition medium containing 5% lipid-depleted fetal bovine serum, and the degrees of TGF-β1 have been evaluated individually within the cells and supernatant utilizing Q-PCR and ELISA. n = 3 unbiased experiments. B HCT-8 cells have been handled because the indication, and the extent of TGF-β1 within the supernatant was detected by ELISA. n = 3 unbiased experiments. C, D Exogenous Rabgef1 or RABGEF1 was overexpressed in ATP6v0a1-suppressing MC38 cells and ATP6V0A1-suppressing HCT-8 cells; 24-OHC (C; n = 4 unbiased experiments) and TGF-β1 (D; n = 3 unbiased experiments) within the supernatant was measured by ELISA. E, F Following the isolation of ER from the management or Atp6v0a1-suppressing MC38 cells transfected with management or Rabgef1-targeted siRNAs, lipids have been extracted and analyzed for levels of cholesterol utilizing the Amplex™ Pink ldl cholesterol assay (E). Furthermore, TGF-β1 ranges within the supernatants have been analyzed by ELISA (F). n = 3 unbiased experiments. G, H ATP6V0A1-suppressing MC38/HCT-8 cells have been transfected with management or Rabgef1/RABGEF1-targeted siRNAs, and 24-OHC (G; n = 3 unbiased experiments) or TGF-β1 (H; n = 4 unbiased experiments) within the supernatant was measured by ELISA. For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing unpaired two-sided Scholar’s t-test. Supply information and actual p-value are supplied as a Supply Information file.
Because the V-ATPase complicated performs a job in vesicle trafficking, we subsequent explored whether or not the regulation of the RABGEF1/TGF-β1 pathway by ATP6V0A1 depends on the modifications within the expression degree of the V-ATPase complicated. As proven in Supplementary Fig. 22, upon the knockdown of ATP6V0A1, ATP6V0A3 (translated by TCIRG1) protein degree was upregulated whereas ATP6V0A2 expression was not considerably modified (Supplementary Fig. 22A, B). ATP6V0A4 is comparatively lowly expressed in each murine (Supplementary Fig. 22A) and human (Supplementary Fig. 1B) CRCs. Furthermore, ATP6V0A1 suppression didn’t alter the protein ranges of ATP6V0C and ATP6V1A (Supplementary Fig. 22A, B), that are related to the V0 and V1 subcomplex ranges of the V-ATPase, respectively. Due to this fact, the modifications in ATP6V0A1 expression might not alter the full expression ranges of the V-ATPase complicated. Furthermore, the suppression of different subtypes of the V0A subunit, reminiscent of ATP6V0A2 and ATP6V0A3, didn’t considerably have an effect on the expression of each RABGEF1 and TGF-β1 (Supplementary Fig. 22C–F), and the extent of ER-derived ldl cholesterol (Supplementary Fig. 22G, H), suggesting that ATP6V0A1 might have distinct capabilities in regulating immune evasion in comparison with the opposite V0A subtypes. Collectively, ATP6V0A1-regulated RABGEF1-dependent ldl cholesterol absorption and TGF-β1 expression don’t depend on the modifications within the expression degree of the V-ATPase complicated or its V0 subcomplex.
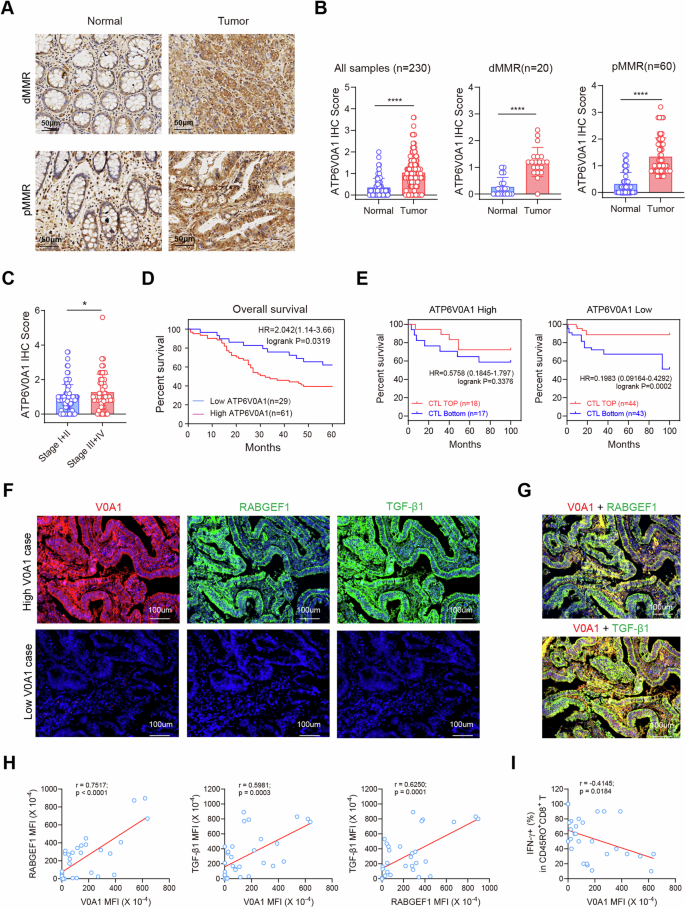
ATP6V0A1 is positively correlated with RABGEF1, TGF-β1 and immunosuppressive TME in medical CRC samples
In line with the immunohistochemical (IHC) evaluation of a human CRC tissue microarray (Fig. 8A, B), ATP6V0A1 exhibited considerably increased expression ranges in tumor tissues in comparison with adjoining non-tumor tissues (paired t-test; p < 0.0001), no matter dMMR and pMMR standing. Furthermore, ATP6V0A1 protein ranges have been considerably increased in sufferers with advanced-stage illness (Phases III + IV) than in sufferers with early-stage CRC (Phases I + II; Fig. 8C). Importantly, ATP6V0A1 protein ranges have been inversely correlated with the general survival of CRC sufferers (Fig. 8D). Moreover, in a Tumor Immune Dysfunction and Exclusion (TIDE) evaluation21 primarily based on the GSE38832 database, excessive infiltration of cytotoxic T lymphocytes (CTLs) was discovered to foretell higher survival in CRCs with low ATP6V0A1 ranges (p = 0.0002), however not in these with excessive ATP6V0A1 ranges (p = 0.3376; Fig. 8E), suggesting the roles of ATP6V0A1 in predicting T-cell dysfunction in CRC. Collectively, ATP6V0A1 is extremely expressed in human CRCs and predicts inactive anti-tumor immunity and poor survival.
A, B Consultant pictures of ATP6V0A1 in tumor tissues and the corresponding peritumoral tissues have been proven for CRC sufferers with dMMR or pMMR (A). Paired t-test evaluation of ATP6V0A1 ranges in all 230 tumor tissues, 20 tumor tissues with dMMR, or 60 tumor tissues with pMMR and their corresponding peritumoral tissues (B). Tumor tissues from 153 sufferers with stage info (C) and 90 sufferers with survival info (D) have been analyzed to match ATP6V0A1 expression in early and late levels and decide the correlation between ATP6V0A1 expression and affected person total survival, respectively. E The prognostic worth of ATP6V0A1 in predicting T-cell dysfunction and affected person survival in CRC was evaluated by the Tumor Immune Dysfunction and Exclusion (TIDE) evaluation primarily based on the GSE38832 database. F–I Paraffin-embedded tumor sections from 32 CRC sufferers have been stained with antibodies towards ATP6V0A1, RABGEF1, TGF-β1, and IFN-γ+CD45RO+CD8+ T cells, and the correlations between ATP6V0A1 and RABGEF1, TGF-β1, or IFN-γ+CD45RO+CD8+ T cells have been analyzed. Consultant immunofluorescence (IF) pictures for ATP6V0A1, RABGEF1, and TGF-β1 expression between high- and low-ATP6V0A1 circumstances have been proven (F). Consultant pictures displaying the co-localization of ATP6V0A1 with RABGEF1 or TGF-β1 in excessive ATP6V0A1 circumstances (G). The correlation between ATP6V0A1 and RABGEF1 expression, ATP6V0A1 and TGF–β1 expression, or RABGEF1 and TGF-β1 expression was analyzed amongst 32 CRC specimens (H). The correlation between ATP6V0A1 expression and CD45RO+CD8+ T-cell effectiveness (IFN-γ expression charge) was analyzed amongst 32 CRC specimens (I). For all experiments, information are proven as imply ± s.e.m; *p < 0.05, ****p < 0.0001. Statistical significance was decided utilizing paired two-sided Scholar’s t-test in (B), unpaired two-sided Scholar’s t-test in (C), and two-sided Correlation check (H, I). MFI imply fluorescence depth. Supply information and actual p-value are supplied as a Supply Information file.
To check the correlations between tumor cell-intrinsic ATP6V0A1, tumor cell-derived TGF-β1, and reminiscence CD8+ T cell effectiveness in medical CRC samples, we analyzed a scRNA-seq dataset primarily based on 23 human CRC tumors and 10 regular samples from the GEO database22. Epithelial cell subpopulations have been chosen from the dataset utilizing a technique described within the strategies and Supplementary Fig. 23A–D. ATP6V0A1 and TGFB1 have been expressed in these epithelial cells and confirmed related distributions within the dot plot generated from dimension discount evaluation (Supplementary Fig. 23E). Importantly, when the epithelial cells have been divided into two teams primarily based on ATP6V0A1 expression, Pearson’s Chi-squared evaluation steered that ATP6V0A1 could be the related issue influencing TGFB1 expression (Supplementary Desk 1). Furthermore, the Wilcoxon rank sum check evaluation revealed considerably increased TGFB1 expression within the ATP6V0A1-positive group in comparison with the ATP6V0A1-negative group (p = 4e-15; Supplementary Fig. 23F). Subsequent, we investigated the correlation between reminiscence CD8+ T cell effectiveness and tumor cell-intrinsic ATP6V0A1 or TGFB1 within the medical samples utilizing a technique described within the strategies, Supplementary Fig. 23G, and Supplementary Desk 2. Among the many 17 tumor samples with three or extra reminiscence CD8+ T cells, epithelial ATP6V0A1 expression correlated positively with epithelial TGFB1 expression (R = 0.47, p = 0.025), and each epithelial ATP6V0A1 (R = −0.54, p = 0.026) and epithelial TGFB1 (R = −0.49, p = 0.046) have been inversely correlated with the cytotoxicity of reminiscence CD8+ T cells (evaluated because the imply worth of GZMA, PRF1, and KLRG1 expression) (Supplementary Fig. 23H–J). Notably, primarily based on information from the TCGA database, excessive ranges of efficient reminiscence CD8+ T cells (indicated by the signature of CD8, EOMES, CD44, GZMA, PRF1, and KLRG1) have been positively correlated with improved total survival in CRC sufferers (Supplementary Fig. 23K).
To strengthen the medical proof supporting the aforementioned outcomes, we carried out an immunofluorescence (IF) assay to analyze the correlation between ATP6V0A1 and RABGEF1, TGF-β1, or reminiscence CD8+ T-cell effectiveness on the paraffin-embedded tumor sections obtained from 32 CRC sufferers. As proven in Fig. 8F, throughout the tumor tissues with ATP6V0A1 expression, each expression of RABGEF1 and TGF-β1 have been comparatively increased within the areas with excessive ATP6V0A1 moderately than these with low ATP6V0A1 (Fig. 8F, higher). Then again, each RABGEF1 and TGF-β1 have been not often detected within the tumor tissues with minimal detection of ATP6V0A1 (Fig. 8F, decrease). Furthermore, important colocalization of ATP6V0A1 and RABGEF1 or TGF-β1 was noticed within the tumor tissues with excessive ATP6V0A1 (Fig. 8G). Importantly, amongst these 32 human CRC specimens, ATP6V0A1 expression was positively correlated with each RABGEF1 and TGF-β1 expression; the expression of RABGEF1 was additionally positively correlated with that of TGF-β1 (Fig. 8H). Moreover, the next expression charge of IFN-γ in CD45RO+ reminiscence CD8+ T cells was noticed within the tumor tissues with low ATP6V0A1 moderately than these with excessive ATP6V0A1 (Supplementary Fig. 24). ATP6V0A1 expression was inversely correlated with the expression charge of IFN-γ in CD45RO+CD8+ T cells within the 32 CRC specimens (Fig. 8I). These findings present medical proof supporting the inhibition of reminiscence CD8+ T cell efficacy by ATP6V0A1 by means of RABGEF1/TGF-β1 signaling in CRC.
Focusing on ATP6V0A1 restores reminiscence CD8+ T-cell-mediated anti-tumor immunity in CRC
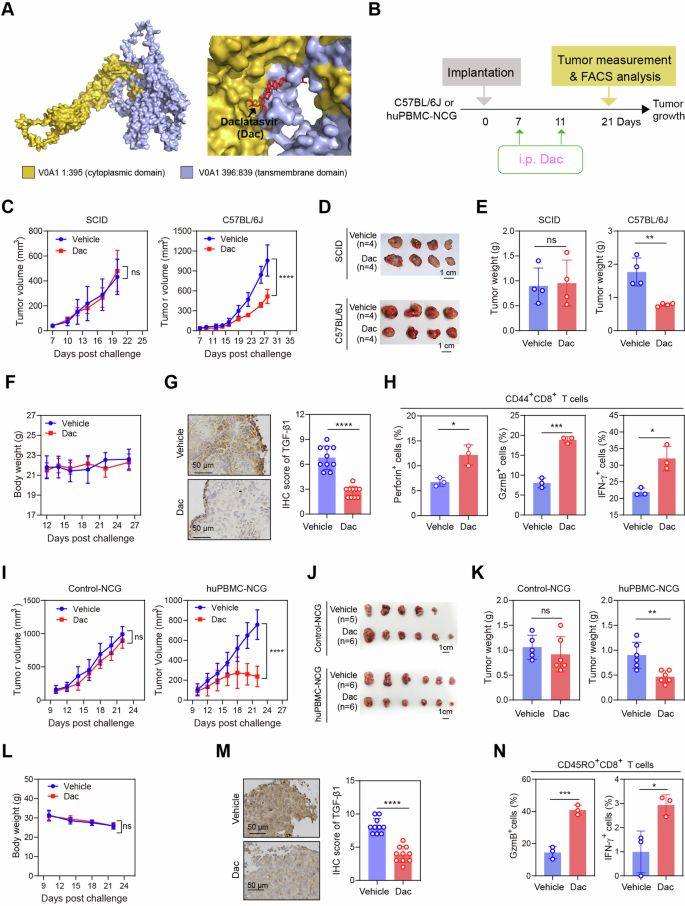
Our outcomes steered that tumor-intrinsic ATP6V0A1 induces immunosuppressive signaling and immune evasion in CRC by reducing the effectiveness of reminiscence CD8+ T cells. Due to this fact, we needed to discover the therapeutic potential of ATP6V0A1 inhibition in CRC animal fashions. First, we used docking-based evaluation of protein-inhibitor interactions to display screen a bunch of FDA-approved small molecular inhibitors for potential inhibitors of ATP6V0A1. We discovered that daclatasvir (Dac), a medical used drug to deal with power genotype I and III hepatitis C virus (HCV) an infection23, was in a position to bind to ATP6V0A1 protein (Fig. 9A and Supplementary Fig. 25A, B). We confirmed the interplay utilizing the mobile thermal shift assay, a way of evaluating drug binding to focus on protein in cells and tissues by detection of modifications within the thermal stability of a protein following ligand discovering24. These assays confirmed that Dac may induce thermal stabilization of ATP6V0A1 protein however not a management protein (β-actin) in MC38 (Supplementary Fig. 25C, D) and HCT-8 cells (Supplementary Fig. 25E, F), confirming the binding of Dac to ATP6V0A1 in these cells. Apparently, Dac didn’t considerably bind to different subtypes of the V0A subunit (Supplementary Fig. 25G), suggesting the specificity of Dac binding to ATP6V0A1. Subsequent, we investigated the flexibility of Dac to disrupt ATP6V0A1-mediated cell capabilities in vivo (Fig. 9B). Apparently, whereas Dac therapy didn’t alter the expansion of MC38 tumors in NOD/SCID mice (Fig. 9C–E), in C57BL/6 J mice it considerably suppressed the expansion of MC38 tumors (Fig. 9C–E), lowered TGF-β1 expression in tumor tissue (Fig. 9G), and activated TME-derived CD44+CD8+ T cells (Fig. 9H). Importantly, Dac therapy additionally considerably suppressed HCT-8 tumor development in human immune-reconstituted huPBMC-NCG mice however not in immunodeficient NCG mice (Fig. 9I–Okay). Furthermore, Dac therapy lowered TGF-β1 expression in tumor tissues (Fig. 9M) and enhanced the activation of CD45RO+CD8+ T cells inside HCT8 tumors (Fig. 9N). Notably, Dac therapy induced neither physique weight reduction (Fig. 9F, L) nor organ toxicity (Supplementary Fig. 26) within the mice, indicating the protection of this therapy.
A A molecular docking strategy predicted Daclatasvir (Dac) as a candidate inhibitor of ATP6V0A1. B Schematic displaying the drug intervention protocol for Dac remedy. C–H NOD/SCID mice and C57BL/6 J mice have been subcutaneously injected with wild-type MC38 cells. Tumor-bearing NOD/SCID mice or C57BL/6 J mice have been then randomized into two teams in line with tumor dimension and handled with automobile or Dac. Common curves for tumor development (C) have been plotted. Images of the tumors (D) and comparisons of tumor weights on day 20 in NOD/SCID mice and day 28 in C57BL/6 J mice (E) are proven. The physique weights of C57BL/6 J mice handled with automobile or Dac have been measured and plotted (F). n = 4 mice per group for C–F. Tissue sections of MC38 tumors from C57BL/6 J mice have been analyzed for TGF-β1 expression (G; n = 10 fields from two mice per group). Tumor-infiltrating CD44+CD8+ T cells from day 28 tumors in C57BL/6 J mice have been analyzed for his or her ranges of effector manufacturing (H; n = 3 mice per group). I–N NCG mice or huPBMC-NCG mice have been subcutaneously injected with HCT-8 wild-type cells. Tumor-bearing NCG mice or huPBMC-NCG mice have been then randomized into two teams in line with tumor dimension and handled with automobile or Dac. n = 5 (Automobile group in Management-NCG mice mannequin) or 6 mice (different teams) in every group. Common curves for tumor development (I) have been plotted. Images of the tumors (J) and comparisons of tumor weights on day 22 (Okay) are proven. The physique weights of huPBMC-NCG mice handled with automobile or Dac have been measured and plotted (L). Sections of HCT-8 tumor tissue from huPBMC-NCG mice have been analyzed for TGF-β1 expression (M; n = 10 fields from two mice per group). Tumor-infiltrating CD45RO+CD8+ T cells from day 22 tumors in huPBMC-NCG mice have been analyzed for his or her ranges of effector manufacturing (N; n = 3 mice per group). For all experiments, information are consultant of three unbiased experiments and proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical significance was decided utilizing strange two-way ANOVA (in C, F, I, L) or unpaired two-sided Scholar’s t-test (in E, G, H, Okay, M, N). Supply data and actual p-value are supplied as a Supply Information file.
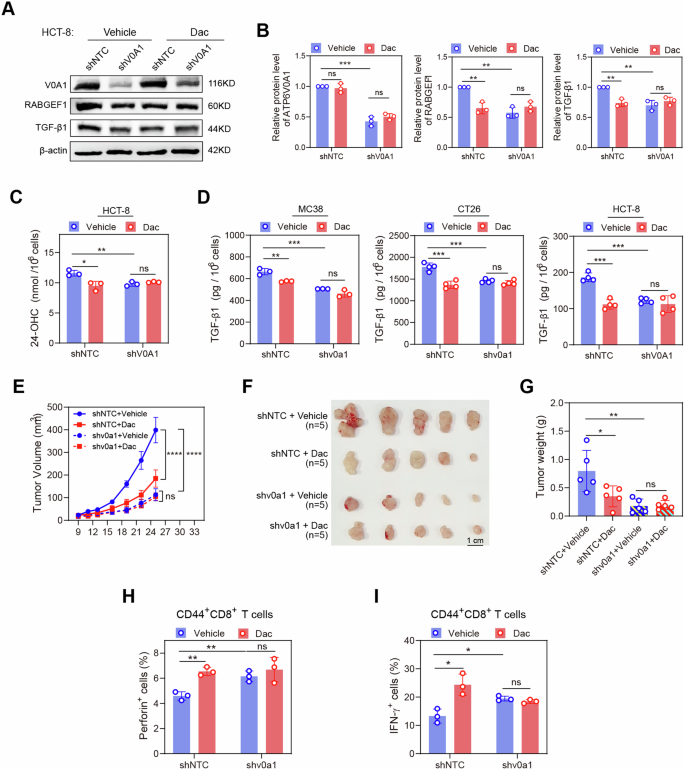
We additional investigated the focusing on specificity of Dac in treating CRC. The in vitro therapy with Dac considerably lowered RABGEF1 expression, 24-OHC manufacturing, and TGF-β1 ranges in management CRC cells however not in ATP6V0A1-deficient CRC cells (Fig. 10A–D). Lastly, Dac therapy suppressed the tumor development (Fig. 10E–G) and restored the activation of CD44+CD8+ T cells (Fig. 10H, I) in management MC38 tumors however not in ATP6V0A1-suppressing MC38 tumors. Collectively, these findings demonstrated that Dac permits the efficient suppression of CRC by focusing on ATP6V0A1.
A–C ATP6V0A1-suppressing HCT-8 cells have been handled with automobile or Dac. The cell lysate was detected with the expression of ATP6V0A1, RABGEF1, and TGF-β1 utilizing western blotting; The presentive blots have been proven (A), and the quantification of those proteins was analyzed primarily based on three unbiased experiments (B). The supernatant was detected by ELISA for 24-OHC degree (C; n = 3 unbiased experiments). D ATP6V0A1-suppressing MC38, CT26, and HCT-8 cells handled with automobile or Dac, and the extent of TGF-β1 within the supernatant was detected by ELISA. n = 3 (MC38) or 4 (CT26 and HCT-8) unbiased experiments. E–I C57BL/6 J mice have been subcutaneously injected with management or Atp6v0a1-suppressing MC38 cells. The mice bearing management MC38 tumors or these bearing Atp6v0a1-suppressing MC38 tumors have been individually randomized into two teams in line with tumor dimension and handled with automobile or Dac. n = 5 mice per group. Common curves for tumor development (E) have been plotted. Images of the tumors (F) and comparisons of tumor weights (G) are proven. Tumor-infiltrating CD44+CD8+ T cells from the above tumors described in E–G have been analyzed for his or her ranges of effector manufacturing (H and I; n = 3 mice per group). For all experiments, information are proven as means ± s.e.m; *p < 0.05, **p < 0.01, ***p < 0.001. Statistical significance was decided utilizing strange two-way ANOVA (in E) or unpaired two-sided Scholar’s t-test (in B, C, D, G, H, I). Supply information and actual p-value are supplied as a Supply Information file.