Introduction
Lung adenosquamous cell carcinoma (ASC)has a low incidence of about 0.4%-4%, making it one of many rarest kinds of lung most cancers (1). ASC is characterised by the presence of each glandular and squamous parts, every constituting at the very least 10% of the tumor (2). This twin histology contributes to the aggressive nature of ASC and poses important therapeutic challenges. The prognosis for ASC sufferers is usually poorer in comparison with these with pure adenocarcinoma or squamous cell carcinoma, reflecting its extra aggressive organic habits and restricted remedy choices. Though immunotherapy improves survival of ASC affected person, in comparison with squamous cell carcinoma and adenocarcinoma, ASC sufferers have a worse prognosis (3–5).
In sufferers with non-small cell lung most cancers (NSCLC) harboring epidermal progress issue receptor (EGFR) mutations, remedy with EGFR tyrosine kinase inhibitors (EGFR-TKIs) at the moment are norm. Third-generation EGFR-TKIs, reminiscent of osimertinib, have change into the present customary of care, notably for sufferers with EGFR T790M resistance mutations (6, 7). EGFR mutations are predominantly present in adenocarcinoma, however they can be detected in 54.8% of ASC sufferers (8). Regardless of the confirmed efficacy of EGFR-TKIs in treating EGFR-mutant NSCLC, the proof for his or her effectiveness in ASC is restricted because of the rarity of the situation and the ensuing shortage of complete research (9). Present remedy pointers and medical trials predominantly concentrate on adenocarcinoma, leaving a spot in tailor-made therapeutic methods for ASC sufferers with EGFR mutations.
On this context, our examine goals to discover the efficacy of EGFR-TKIs in sufferers with EGFR-mutant ASC by a retrospective evaluation and pooled knowledge from printed research. We search to offer insights into the medical outcomes and potential advantages of EGFR-TKIs remedy on this distinctive affected person inhabitants, thereby addressing a important hole within the administration of ASC.
Sufferers and strategies
Sufferers
Between January 2009 and April 2022, we collected medical knowledge on ASC sufferers handled with EGFR-TKI at West China Hospital. All sufferers underwent bronchofiberscope or percutaneous lung biopsies, adopted by immunohistochemistry (IHC) for pathological affirmation. These retrospective analyses had been carried out with knowledgeable consent from every affected person.
We searched PUBMED for all publications describing the usage of EGFR-TKI in superior or recurrent EGFR mutant ASC sufferers for additional analysis into its efficacy. There have been three topic headings used throughout the search interval of 2005 to 2022: lung most cancers, mutation, and EGFR. Journals and publications weren’t restricted by the technique, however abstracts of conferences weren’t accepted. An analysis of EGFR-TKIs used to deal with superior or recurrent ASC sufferers harboring EGFR mutations was included on this examine. The selection was restricted to researches printed within the English journal. EGFR-TKI remedy was supplied to sufferers who met all three standards: (1) superior or recurrent ASC, (2) EGFR mutation, and (3) acceptance of EGFR-TKI remedy (erlotinib 150mg/day, gefitinib 250mg/day, icotinib 125mg tid or dacomitinib 45mg/day). Knowledge, reminiscent of EGFR mutation sort, EGFR-TKI line, and remedy with EGFR-TKI, had been collected as baseline components. The authors had been requested for knowledge that wasn’t included within the article.
Take a look at methodology for EGFR mutation
Within the retrospective knowledge, tissues that had been embalmed or freshly harvested had been used to extract DNA. The mutations in EGFR had been recognized utilizing a quantitative PCR evaluation utilizing the Amplification Refractory Mutation System.A EGFR mutation was detected utilizing the protocol represented in every examine, in response to the printed knowledge.
Scientific assessments
The Response Analysis Standards in Stable Tumors had been used to judge the efficacy of the EGFR-TKI focused remedy. There have been 4 kinds of responses: progressive illness (PD), secure illness (SD), partial response (PR), and full response (CR). The target response fee (ORR) is decided by dividing the proportion of sufferers who had been CR or PR by all sufferers. CR, PR, and SD sufferers had been divided by complete sufferers to find out the illness management fee (DCR). A prognosis of development free survival (PFS) was calculated from the start of remedy to the onset of PD. We additionally calculated general survival (OS) from the second remedy started till loss of life. It was on July 22, 2022, that the final follow-up go to was carried out. In statistical evaluation, sufferers who didn’t progress or had been alive had been censored on July 22, 2022.
Statistical strategies
Qualitative variables had been illustrated as the way in which of absolute and share quantities, whereas steady variables had been illustrated as medians with ranges. With a purpose to conduct the survival evaluation, Kaplan-Meier strategies had been used. An univariate evaluation of log-rank exams was carried out to be able to decide which prognostic components have an effect on PFS and OS. A multivariate evaluation was performed by utilizing Cox regression. The importance of P values is decided by utilizing 0.05. Analyses had been performed utilizing SPSS model 22.0.
Outcomes
Affected person traits
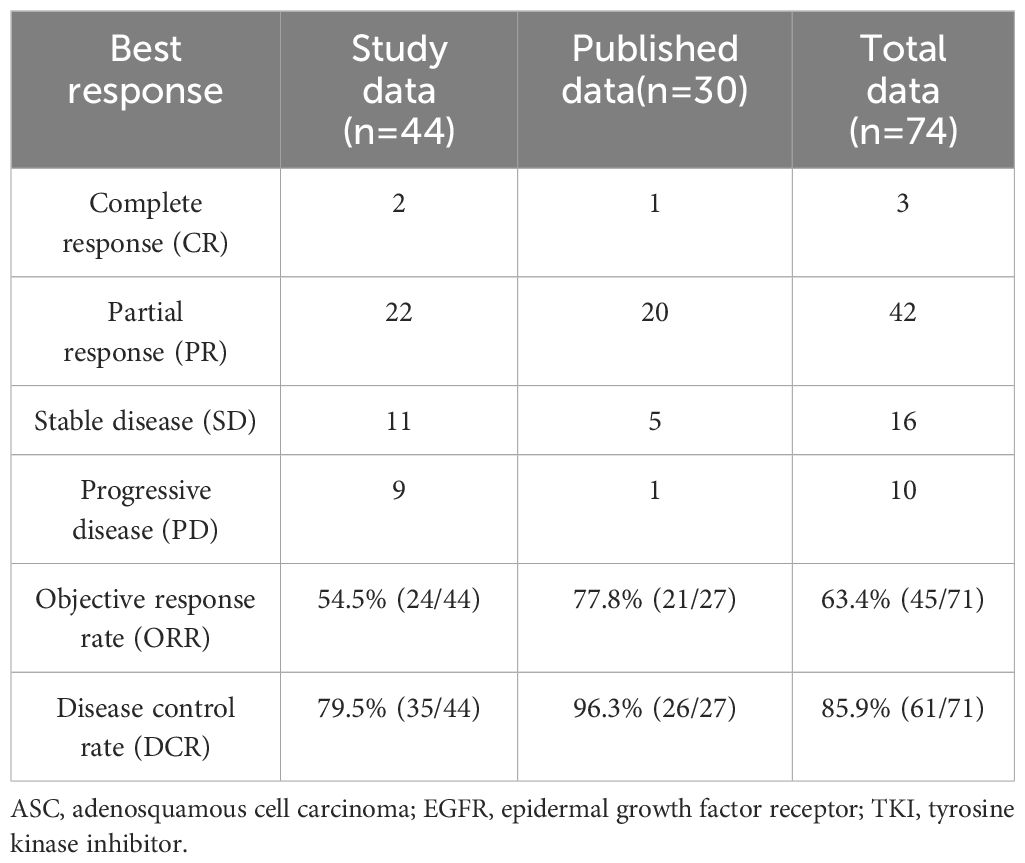
EGFR-TKI remedy was administered to 44 ASC sufferers with EGFR mutations on the two most cancers facilities for the needs of assessing efficacy. Of the 44 ASC sufferers, there have been 22 females and 22 males. Age vary was 34-82 years (median 60.5 years). There have been 17 sufferers with a historical past of smoking. Among the many sufferers, 20 had a mutation in exon 19 (19-DEL), 21 had a mutation in exon 21 (L858R), whereas 3 had a uncommon delicate mutation (G719X, L861Q). As a first-lines remedy, 27 sufferers had been handled with EGFR-TKI, and 17 sufferers had been handled in a second or extra line of remedy. There have been 11 sufferers handled with erlotinib, 21 sufferers handled with gefitinib, 11 sufferers handled with icotinib, and 1 affected person handled with dacomitinib (Desk 1).
Efficacy of EGFR-TKI
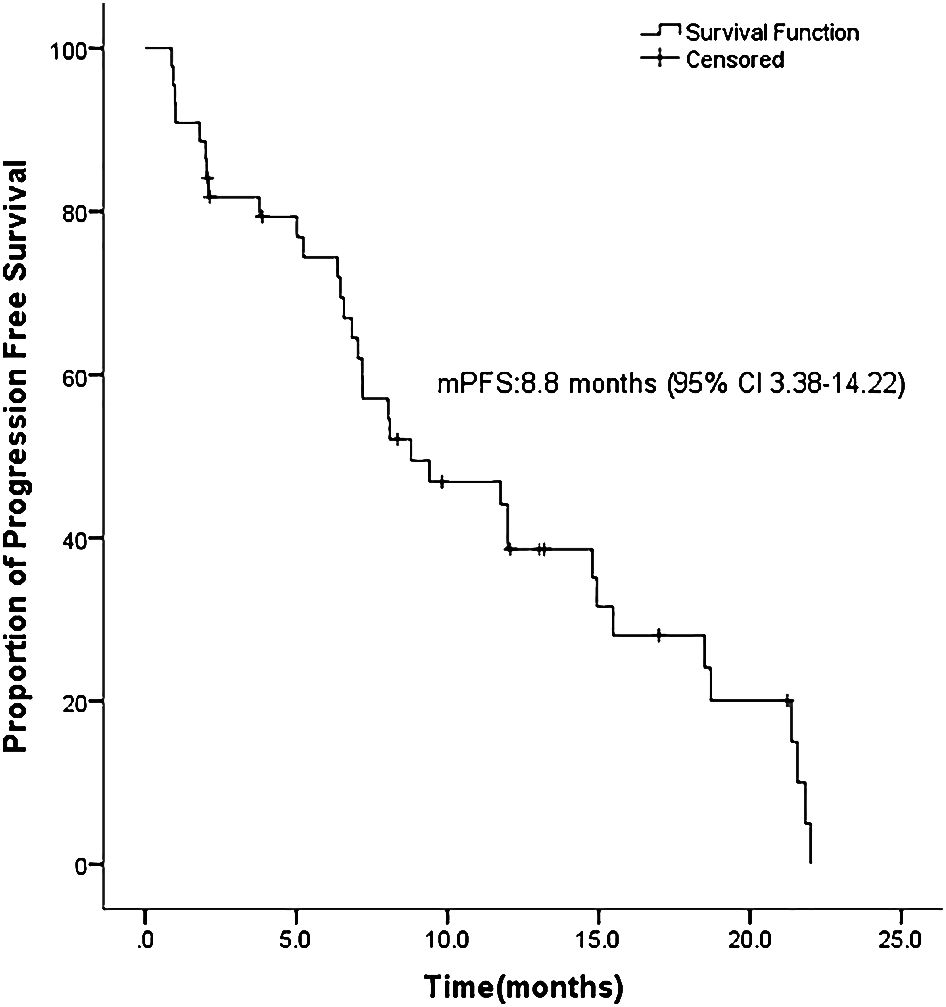
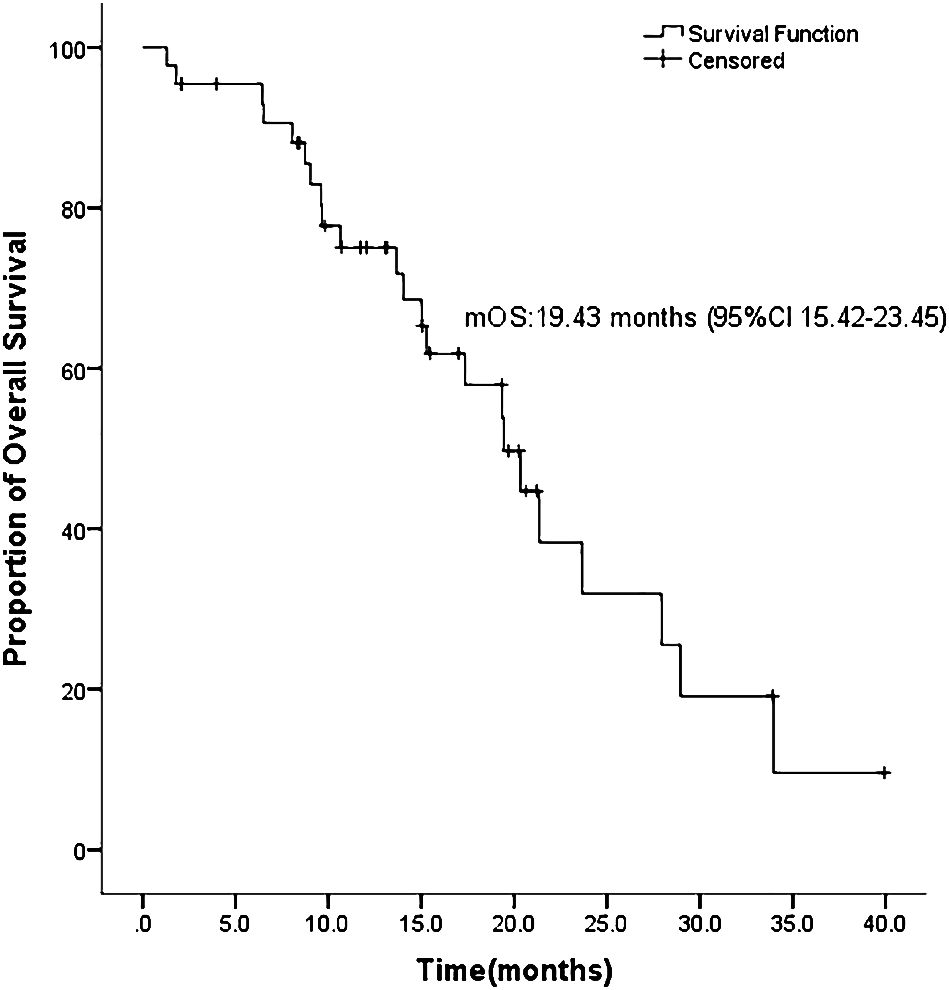
ASCs with EGFR mutations responded to EGFR-TKI with 2 CRs, 22 PRs, 11 SDs, and 9 PDs. The ORR was 54.5% and the DCR was 79.5% for the 44 sufferers (Desk 2). Ten sufferers had not but progressed, whereas 21 sufferers had been nonetheless alive on July 22, 2022. Determine 1 exhibits the mPFS was 8.8 months (95% CI 3.38-14.22), and Determine 2 exhibits the mOS of 19.43 months (95% CI 15.42-23.45).
Pooled evaluation
A complete of 30 sufferers who met the inclusion standards from eleven analysis research had been included on this examine (10–20). The 11 researches consisted of 8 retrospective research and three case reviews. A complete of eight of those researches had been performed in East Asian international locations. 13 sufferers had been from western international locations. Definitive knowledge of age, gender, smoking standing, EGFR mutation sort, traces of EGFR-TKI and EGFR-TKI remedy could possibly be extracted in 21 (70.0%), 30 (100.0%), 28 (93.3%), 30 (100.0%), 22 (73.3%) and 23 (76.7%) of the 30 sufferers, respectively (Tables 1, 3, 4).
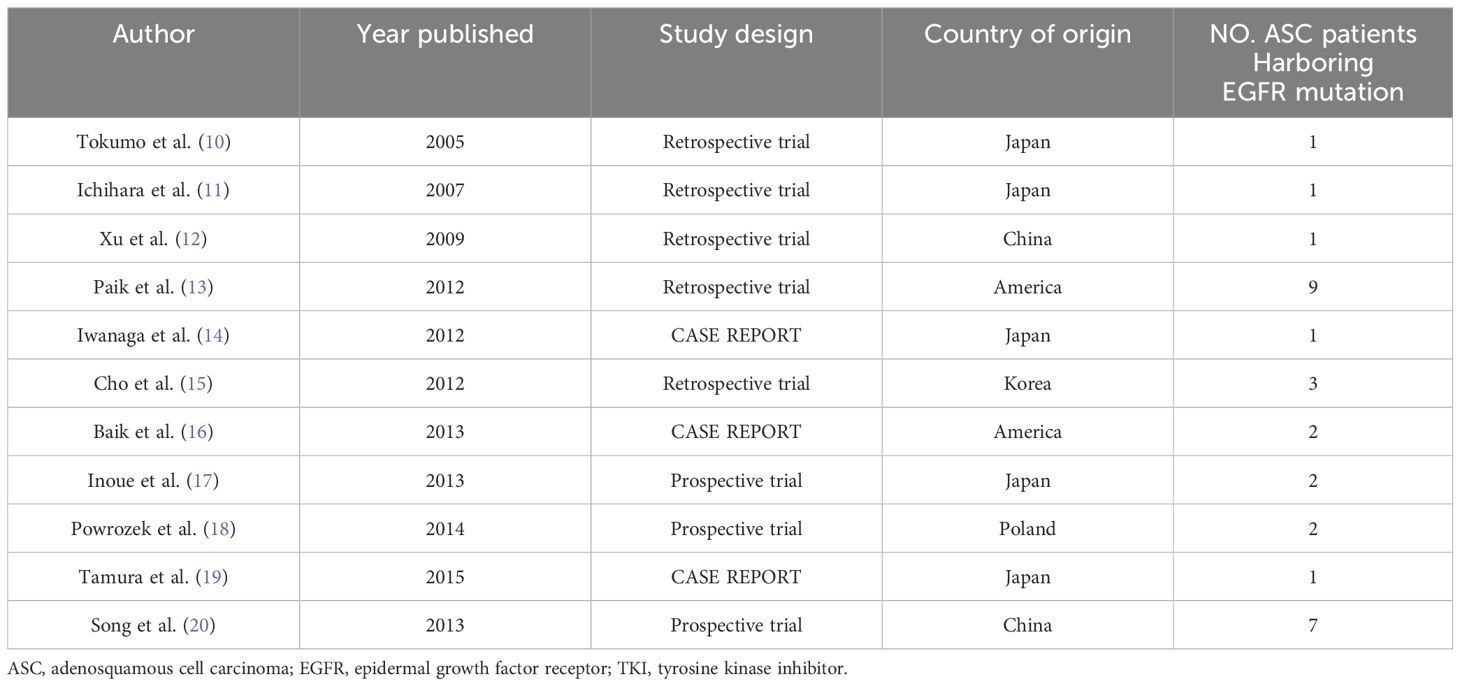
Desk 3 The 11 printed reviews which we might extract the info of recurrent or superior ASC sufferers who had EGFR mutation and had been handled with EGFR-TKI.
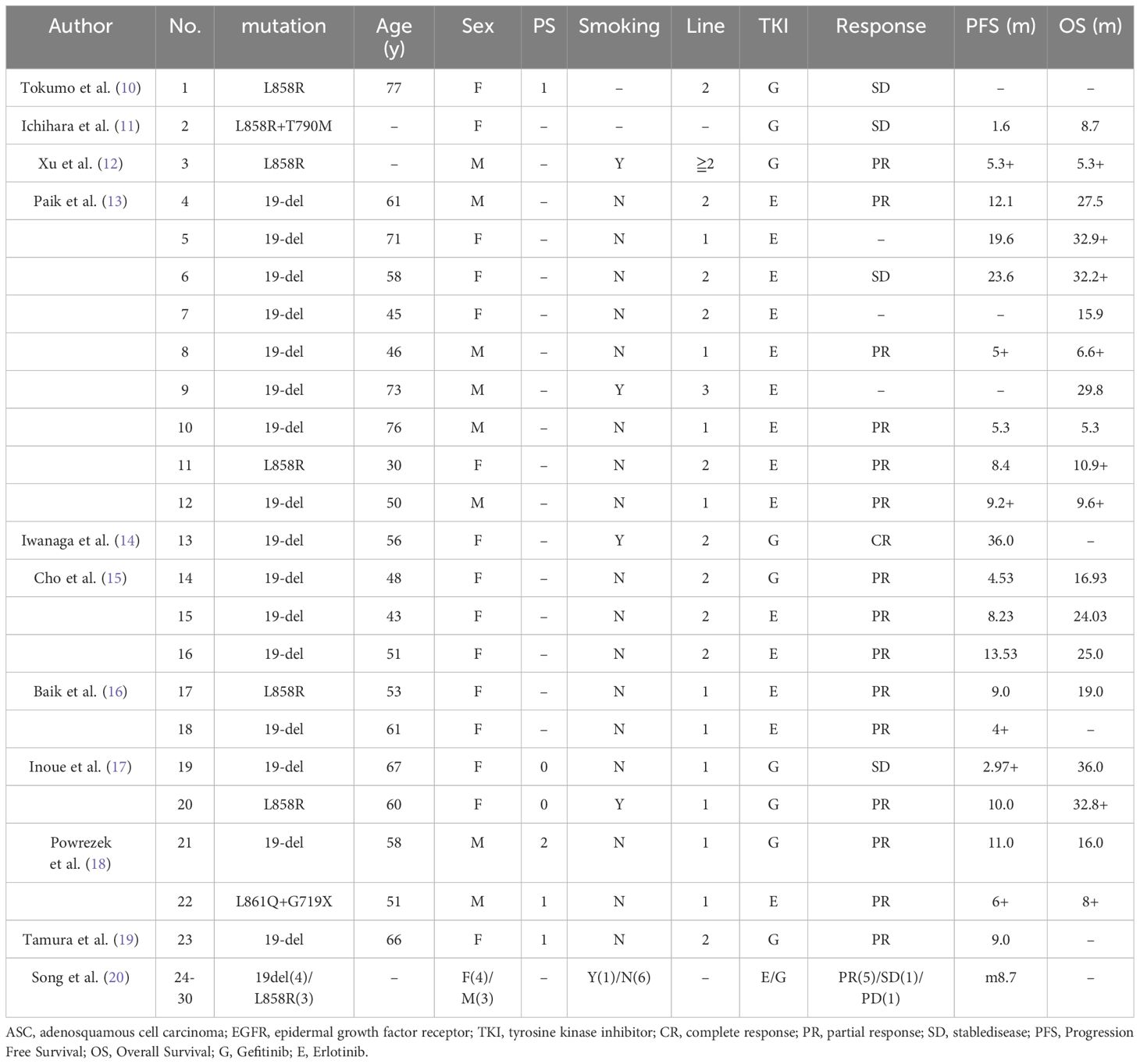
Desk 4 Particular person affected person knowledge of the ASC sufferers with EGFR mutations extracted from the 11 research that evaluated the efficacy of EGFR-TKI for ASC sufferers with EGFR mutations.
Lastly, we pooled knowledge from 74 sufferers. Ages ranged from 30 to 82 years (median 58.5). Gender and smoking historical past had been: male (33/74, 44.6%), feminine (41/74, 55.4%); never-smoker (50/72, 69.4%), smoker (22/72, 30.6%); There have been 40 sufferers (40/74, 54.0%) with exon 19 deletion, 29 sufferers (29/74, 39.2%) with L858R mutation and 4 sufferers (4/74, 5.4%) with uncommon delicate mutation (G719X, L861Q). One affected person (1.4%) who had each a resistant mutation (T790M) and delicate mutation (L858R) was excluded within the evaluation of PFS and OS. Twenty-five (25/67, 37.3%) sufferers obtained erlotinib, 30 sufferers (30/67, 44.8%) obtained gefitinib, 11 sufferers (11/67, 16.4%) obtained icotinib and 1 sufferers (1/67, 1.5%) obtained dacomitinib. In 37 circumstances (37/66, 56.1%), EGFR-TKI was used as the primary line of remedy whereas in 29 circumstances (29/66, 43.9%), second or extra traces of remedy with EGFR-TKI had been used. (Desk 1).
There are 27 sufferers whose tumor responses had been recognized from printed analysis. In complete, 71 sufferers had been evaluated for response. There have been three sufferers with CR, 42 sufferers with PR, 16 sufferers with SD, and 10 sufferers with PD. It had an ORR of 63.4% (45/71) and DCR of 85.9% (61/71) (Desk 2).
19 sufferers with PFS had been recognized in printed analysis. In complete, 63 sufferers had been analyzed for PFS. All sufferers had a mPFS of 10.00 months (95% CI 6.73-13.27). Exon 19 deletion sufferers had a mPFS of 11.00 months (95% CI 6.70-15.30), whereas exon 21 L858R mutation sufferers had a mPFS of 10.00 months (95% CI 5.89-14.11) (P=0.771). In comparison with uncommon delicate mutations (G719X, L861Q) sufferers, exon 19 deletion sufferers or exon 21 L858R mutation sufferers had an extended mPFS (11.00 months vs. 2.10 months, P=0.005; 10.00 months vs. 2.10 months, P=0.019). Univariate evaluation didn’t present important correlations between the info sources, age, gender, smoking standing, EGFR-TKI traces, and EGFR-TKI remedy and PFS. Multivariate evaluation revealed no important correlation between medical options and PFS (Desk 5).
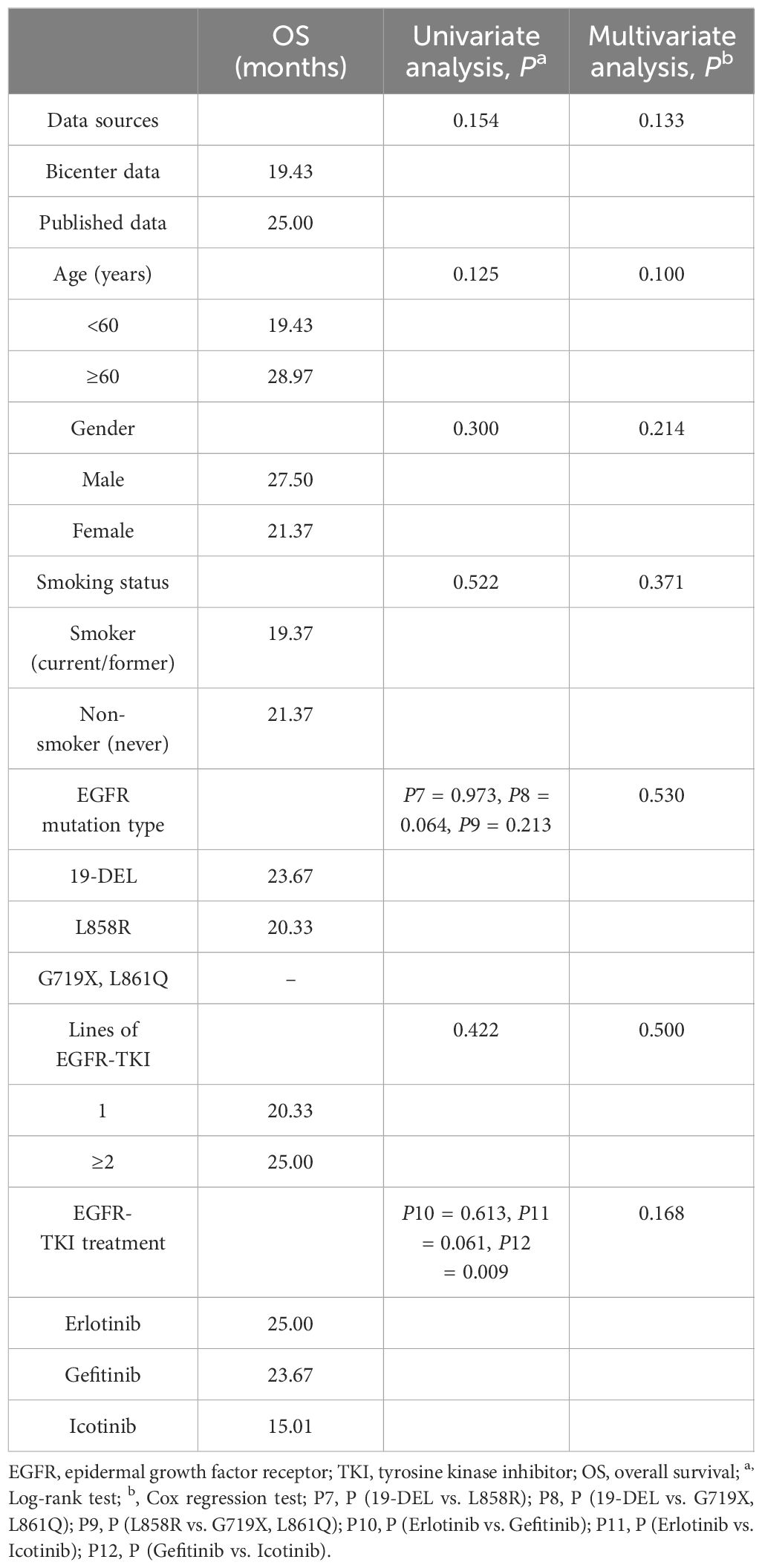
The info of OS was extracted in 18 sufferers from the printed researches. The pooled evaluation of OS included 62 sufferers. The mOS was 21.37 months (95% CI 16.01–26.73). Exon 19 deletion sufferers had a mOS of 23.67 months, whereas exon 21 L858R mutation sufferers had a mOS of 20.33 months (P=0.973). In univariate evaluation, erlotinib remedy led to an extended OS in contrast with icotinib remedy (25.00 months vs. 15.01 months, P=0.061); In univariate evaluation, a mOS of 23.67 months was seen in sufferers handled with gefitinib in contrast with 15.01 months in sufferers handled with icotinib (P=0.009); Univariate analyses confirmed no important correlation between the info sources, age, gender, smoking standing, and contours of EGFR-TKIs and OS. In multivariate evaluation, no medical options had been discovered to be correlated considerably with OS (Desk 6).
Dialogue
Literature research regarding EGFR-TKI sensitivity in ASC harboring EGFR mutation are restricted because of the low incidence of ASC in lung most cancers. Consequently, there may be not sufficient proof to help the efficacy of EGFR-TKI within the remedy of ASC. In our pooled evaluation, there was an ORR of 63.4% and DCR of 85.9% in ASC sufferers handled with EGFR-TKI, and a mPFS of 10.00 months and a mOS of 21.37 months in these sufferers. Therefore, ASC containing mutant EGFR are successfully handled with EGFR-TKI. In the meantime, EGFR mutation will be detected in 54.8% of ASC sufferers which demonstrated that the mutation fee is parallel to ADC (8, 21). As EGFR mutations are extremely prevalent in ASC sufferers and EGFR-TKIs are extremely efficient, we suggest routine EGFR mutation testing for all ASC sufferers. To our data, our examine represents one of many largest research of EGFR-TKI efficacy in lung ASC sufferers harboring mutations in EGFR. We imagine this knowledge deserves medical reference.
ADC has been efficiently handled with EGFR-TKI in earlier medical research, with ORRs of 70-85% and mPFS of 8-13 months (7, 22). Earlier analysis has indicated that ASC sufferers with EGFR mutations obtain mPFS of 9.3 months when handled with first-generation EGFR-TKI (9). On account of our examine, lung ASC had an ORR of 63.4% and a median PFS of 10.00 months. Our examine exhibits that lung ASC with EGFR mutations reply successfully to EGFR-TKI remedy, albeit with a barely decrease efficacy in comparison with pure adenocarcinomas. The distinct organic habits of ASC, which incorporates each squamous and glandular parts, may contribute to the variations in remedy outcomes (3). The heterogeneity throughout the tumor could influence the response to EGFR-TKI, as adenocarcinoma and squamous parts could reply in another way to remedy. Apart from, the variability within the molecular profile of ASC tumors, as in comparison with pure adenocarcinomas, may also be an element (8). This variability might affect the tumor’s response to EGFR-TKI remedy. Our examine suggests a necessity for additional analysis to discover the molecular mechanisms behind the differential response of ASC and pure adenocarcinomas to EGFR-TKI remedy.
In line with our analysis, in sufferers with uncommon delicate mutations (G719X, L861Q), the distinction in PFS was statistically important when in comparison with sufferers with deletion of exon 19 or exon 21 L858R mutations. Nevertheless, as a result of there have been solely 4 sufferers with uncommon delicate mutation, the end result wanted to be additional testified by extra researches. Additional, earlier research have proven that ADC sufferers with a L858R mutation in exon 21 of EGFR have considerably decrease efficacy with EGFR-TKI remedy than sufferers in exon 19 of EGFR (23). Nevertheless, sufferers with deletions in exon 19 and exon 21 L858R mutations had comparable PFS (11.0 vs 10.0 months, P=0.771) and OS (23.67 vs. 20.33 months, P=0.973). The reason for this distinction must additional examine. Our examine primarily targeted on the preliminary efficacy of EGFR-TKI in ASC sufferers. EGFR-TKI acquired resistance in lung ASC is step by step changing into a analysis hotspot (24). The development and resistance mechanisms, together with the frequency of T790M mutations, are undoubtedly essential and future research specializing in this side would certainly be worthwhile. Bsides, the efficacy in lung ASC of third era TKI reminiscent of osimertinib and ceritinib nonetheless wants additional examine (25, 26). Immunotherapy has proven promising prospects within the remedy of lung ASC (5).
In a single printed analysis, 55 ASC sufferers had been demonstrated twin differentiation with various proportions of ADC and SCC by utilizing the microdissection (27). There’s a pity that pathology was unable to find out which of 44 sufferers carried squamous cell carcinomatous and adenocarcinomatous parts. Furthermore, some researches found that the an identical EGFR mutation patterns within the squamous cell carcinomatous and the adenocarcinomatous parts in every affected person, indicated the monoclonality of the 2 tumor parts in ASC sufferers (28, 29). This conclusion was additionally testified by different researches (3). For the reason that an identical EGFR mutation patterns occurred within the squamous cell carcinomatous and the adenocarcinomatous parts of ASC, It could be the proportion of two tumor parts in EGFR mutant ASC sufferers that determines the efficacy of EGFR-TKI in EGFR mutant ASC sufferers. The predominance of 1 part over the opposite might probably have an effect on the remedy outcomes. As well as, the therapeutic benefits on adenocarcinoma parts of TKI could generate the withering of the adenocarcinomatous parts of ASC, whereas the squamous cell carcinomatous of ASC achieve a quantitative benefit (30). Researchers at our most cancers heart are investigating how the ratio of those two tumor parts and EGFR-TKI efficacy are associated.
In NSCLC, particularly in metastatic illness, small biopsy samples could make it troublesome to precisely differentiate between squamous cell carcinoma and ASC (1). This distinction is essential because it impacts remedy selections. Molecular testing, together with EGFR mutation evaluation, can play a important position in figuring out sufferers who may profit from focused therapies (31). That is notably related in circumstances the place histological classification is unsure. Given the histological overlap between squamous tumors and ASC, molecular testing gives a extra exact strategy to determine the tumor’s traits, thus guiding applicable remedy (3). The American Society of Scientific Oncology (ASCO) emphasizes the necessity for complete molecular profiling in NSCLC. By incorporating molecular testing, clinicians can higher tailor remedy methods to particular person affected person wants, particularly for these with uncommon or atypical NSCLC subtypes like ASC.
It’s essential to illustrate the restrictions of this examine. Among the many chosen printed research, inclusion standards and check strategies for EGFR mutations had been completely different, and medical traits weren’t fully described. Furthermore, the retrospective nature was one other limitation of this analysis. The low incidence of ASC in lung most cancers, nonetheless, makes our analysis fairly important as effectively.
In conclusion, this examine which concerned all obtainable knowledge, together with knowledge collected from our most cancers facilities of China and that pooled from earlier research, and recognized the medical profiles of EGFR-TKI software, steered that EGFR-TKI was discovered to be an efficient remedy in ASC harboring mutations in EGFR. Moreover, the examine recommends that EGFR mutation testing be performed routinely on all lung ASC sufferers.
Knowledge availability assertion
The unique contributions introduced within the examine are included within the article/supplementary materials. Additional inquiries will be directed to the corresponding creator.
Ethics assertion
The research involving people had been authorized by West China hospital’s Institutional Evaluation Board. The research had been performed in accordance with the native laws and institutional necessities. Written knowledgeable consent for participation on this examine was supplied by the contributors’ authorized guardians/subsequent of kin.
Creator contributions
XX: Conceptualization, Knowledge curation, Writing – authentic draft. WD: Knowledge curation, Writing – authentic draft. YZ: Supervision, Validation, Writing – authentic draft. YYL: Investigation, Software program, Writing – authentic draft. MY: Formal evaluation, Methodology, Sources, Writing – authentic draft. YML: Writing – overview & enhancing.
Funding
The creator(s) declare that no monetary help was obtained for the analysis, authorship, and/or publication of this text.
Acknowledgments
We thank Dr. Baohui Han, MD, Division of Pulmonary, Shanghai Chest Hospital, the Individuals’s Republic of China, Dr. Tomasz POWRÓZEK, MD, Pneumonology, Oncology and Allergology Division, Medical College of Lublin, Poland, Dr. Christina Baik, MD, Thoracic, Head and Neck Medical Oncology, Seattle Most cancers Care Alliance, Fred Hutchinson Most cancers Analysis Heart, College of Washington, Dr. Akira Inoue, MD, the Division of Respiratory Drugs, Tohoku College Hospital, Seiryomachi, Aobaku, Sendai, Japan and Dr. Younger-Chul Kim, MD, Lung most cancers clinic, Pulmonary Drugs, Chonnam Nationwide College Medical College, Hwasun Hospital, Jeollanam-do, South Korea for offering the info of their sufferers.
Battle of curiosity
The authors declare that the analysis was performed within the absence of any industrial or monetary relationships that could possibly be construed as a possible battle of curiosity.
Writer’s word
All claims expressed on this article are solely these of the authors and don’t essentially signify these of their affiliated organizations, or these of the writer, the editors and the reviewers. Any product that could be evaluated on this article, or declare that could be made by its producer, will not be assured or endorsed by the writer.
References
2. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: influence of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
PubMed Summary | CrossRef Full Textual content | Google Scholar
3. Lin G, Li C, Li PS, Fang WZ, Xu HP, Gong YH, et al. Genomic origin and EGFR-TKI remedies of pulmonary adenosquamous carcinoma. Ann Oncol. (2020) 31:517–24. doi: 10.1016/j.annonc.2020.01.014
PubMed Summary | CrossRef Full Textual content | Google Scholar
4. Li C, Zheng XB, Li PS, Wang HJ, Hu J, Wu L, et al. Heterogeneity of tumor immune microenvironment and real-world evaluation of immunotherapy efficacy in lung adenosquamous carcinoma. Entrance Immunol. (2022) 13. doi: 10.3389/fimmu.2022.944812
5. Wei J, Xiang J, Hao Y, Si J, Gu X, Xu M, et al. Scientific outcomes of immune checkpoint inhibitor remedy for superior lung adenosquamous carcinoma. J Thorac Illness. (2023) 15:260–9. doi: 10.21037/jtd
6. Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in sufferers with superior EGFR mutation-positive non-small-cell lung most cancers: analyses from the part III, randomized, open-label, ENSURE examine. Ann Oncol. (2015) 26:1883–9. doi: 10.1093/annonc/mdv270
PubMed Summary | CrossRef Full Textual content | Google Scholar
7. Tian X, Gu T, Lee M-H, Dong Z. Problem and countermeasures for EGFR focused remedy in non-small cell lung most cancers. Biochim Biophys Acta (BBA) – Rev Most cancers. (2022) 1877:188645. doi: 10.1016/j.bbcan.2021.188645
8. Wang H, Liu J, Zhu S, Miao Okay, Li Z, Qi X, et al. Complete analyses of genomic options and mutational signatures in adenosquamous carcinoma of the lung. Entrance Oncol. (2022) 12:945843. doi: 10.3389/fonc.2022.945843
PubMed Summary | CrossRef Full Textual content | Google Scholar
9. Hu MJ, Zhang B, Xu JL, Wang SY, Zhao YM, Zhang LL, et al. Scientific outcomes of various generations of EGFR tyrosine kinase inhibitors in superior lung adenosquamous carcinoma. Mol Analysis Ther. (2019) 23:773–9. doi: 10.1007/s40291-019-00425-x
10. Tokumo M, Toyooka S, Kiura Okay, Shigematsu H, Tomii Okay, Aoe M, et al. The connection between epidermal progress issue receptor mutations and clinicopathologic options in non-small cell lung cancers. Clin Most cancers Res. (2005) 11:1167–73. doi: 10.1158/1078-0432.1167.11.3
PubMed Summary | CrossRef Full Textual content | Google Scholar
11. Ichihara S, Toyooka S, Fujiwara Y, Hotta Okay, Shigematsu H, Tokumo M, et al. The influence of epidermal progress issue receptor gene standing on gefitinib-treated Japanese sufferers with non-small-cell lung most cancers. Int J Most cancers. (2007) 120:1239–47. doi: 10.1002/ijc.22513
PubMed Summary | CrossRef Full Textual content | Google Scholar
12. Xu JM, Han Y, Duan HQ, Gao EM, Zhang Y, Liu XQ, et al. EGFR mutations and HER2/3 protein expression and medical final result in Chinese language superior non-small cell lung most cancers sufferers handled with gefitinib. J Most cancers Res Clin Oncol. (2008) 135:771–82. doi: 10.1007/s00432-008-0512-1
PubMed Summary | CrossRef Full Textual content | Google Scholar
13. Paik PK, Varghese AM, Sima CS, Moreira AL, Ladanyi M, Kris MG, et al. Response to erlotinib in sufferers with EGFR mutant superior non–small cell lung cancers with a squamous or squamous-like part. Mol Most cancers Ther. (2012) 11:2535–40. doi: 10.1158/1535-7163.MCT-12-0163
PubMed Summary | CrossRef Full Textual content | Google Scholar
14. Iwanaga Okay, Sueoka-Aragane N, Nakamura T, Mori D, Kimura S. The long-term survival of a affected person with adenosquamous lung carcinoma harboring EGFR-activating mutations who was handled with gefitinib. Inside Med. (2012) 51:2771–4. doi: 10.2169/internalmedicine.51.7428
15. Cho S-H, Park LC, Ji JH, Park S, Hwang DW, Lee JY, et al. Efficacy of EGFR tyrosine kinase inhibitors for non-adenocarcinoma NSCLC sufferers with EGFR mutation. Most cancers Chemother Pharmacol. (2012) 70:315–20. doi: 10.1007/s00280-012-1876-0
PubMed Summary | CrossRef Full Textual content | Google Scholar
17. Inoue A, Kobayashi Okay, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Up to date general survival outcomes from a randomized part III trial evaluating gefitinib with carboplatin–paclitaxel for chemo-naïve non-small cell lung most cancers with delicate EGFR gene mutations (NEJ002). Ann Oncol. (2013) 24:54–9. doi: 10.1093/annonc/mds214
PubMed Summary | CrossRef Full Textual content | Google Scholar
18. Powrózek T, Krawczyk P, Ramlau R, Sura S, Wojas-Krawczyk Okay, Kucharczyk T, et al. EGFRgene mutations in sufferers with adenosquamous lung carcinoma. Asia-Pacific J Clin Oncol. (2014) 10:340–5. doi: 10.1111/ajco.12177
19. Tamura T, Kagohashi Okay, Satoh H. Profitable afatinib remedy after resistance to EGFR-TKI in a affected person with superior adenosquamous cell lung most cancers. Oncol Res Deal with. (2015) 38:316–7. doi: 10.1159/000431351
PubMed Summary | CrossRef Full Textual content | Google Scholar
20. Tune Z, Lin B, Shao L, Zhang Y. Therapeutic efficacy of gefitinib and erlotinib in sufferers with superior lung adenosquamous carcinoma. J Chin Med Assoc. (2013) 76:481–5. doi: 10.1016/j.jcma.2013.05.007
PubMed Summary | CrossRef Full Textual content | Google Scholar
21. Wang T, Zhou J, Wang Y, Zheng Q, Lin Z, Li G, et al. Clinicopathological traits and prognosis of resectable lung adenosquamous carcinoma: a population-based examine of the SEER database. Japanese J Clin Oncol. (2022). 52:1456. doi: 10.1093/jjco/hyac096
22. Nishihara S, Yamaoka T, Ishikawa F, Ohmori T, Ando Okay, Kusumoto S, et al. Numerous mechanisms of resistance in opposition to osimertinib, a third-generation EGFR-TKI, in lung adenocarcinoma cells with an EGFR-activating mutation. Cells. (2022) 11. doi: 10.3390/cells11142201
PubMed Summary | CrossRef Full Textual content | Google Scholar
23. Yang JC-H, Wu Y-L, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): evaluation of general survival knowledge from two randomised, part 3 trials. Lancet Oncol. (2015) 16:141–51. doi: 10.1016/S1470-2045(14)71173-8
PubMed Summary | CrossRef Full Textual content | Google Scholar
24. Wu YH, Zhang Okay, Guan JX, Wu WB, Zhang J, Chen HG. Therapy with anlotinib after chemotherapy and EGFR-TKI resistance in lung adenosquamous carcinoma with concurrent EGFR and PIK3CA mutations: A case report and literature overview. Most cancers Handle Res. (2021) 13:7047–53. doi: 10.2147/CMAR.S326094
25. Kim SY, Kim KE, Kim Y, Chung CK. A affected person with a lung adenosquamous carcinoma harboring a de novo T790M mutation and big nonbacterial vegetative growths efficiently handled with osimertinib: A case report. Thorac Most cancers. (2023) 14:1530–3. doi: 10.1111/1759-7714.14896
PubMed Summary | CrossRef Full Textual content | Google Scholar
26. Mai SX, Wang Y, Wang XM, Yang W, Gao HC, Xu ZN, et al. Neoadjuvant ceritinib remedy in ALK-rearranged domestically superior adenosquamous carcinoma: A case report. Thorac Most cancers. (2022) 13:2275–8. doi: 10.1111/1759-7714.14558
PubMed Summary | CrossRef Full Textual content | Google Scholar
27. Cheng C, Luo Z, Xiong W, Shi ZQ, Tan H. Epidemiology and survival outcomes in adenosquamous carcinoma: a population-based examine. Int J Colorectal Illness. (2022) 37:1581–92. doi: 10.1007/s00384-022-04198-4
28. Toyooka S, Yatabe Y, Tokumo M, Ichimura Okay, Asano H, Tomii Okay, et al. Mutations of epidermal progress issue receptor andK-rasgenes in adenosquamous carcinoma of the lung. Int J Most cancers. (2006) 118:1588–90. doi: 10.1002/ijc.21500
PubMed Summary | CrossRef Full Textual content | Google Scholar
29. Shi XH, Wu HW, Lu JL, Duan HL, Liu XG, Liang ZY. Screening for main driver oncogene alterations in adenosquamous lung carcinoma utilizing PCR coupled with next-generation and Sanger sequencing strategies. Sci Rep-Uk. (2016) 6:22297. doi: 10.1038/srep22297
30. Ge-Ge L, Cuicui G, Leiqiang L, Yongcang T, Jiangang M, Yiwen O, et al. Case report: A case report and literature overview about Pathological transformation of lung adenosquamous cell carcinoma. Entrance Oncol. (2022) 12. doi: 10.3389/fonc.2022.1029679
PubMed Summary | CrossRef Full Textual content | Google Scholar