STING agonists attenuate bone cancer-induced ache and restore locomotor operate
We first sought to find out whether or not activation of STING through systemic administration of DMXAA might present long-term therapeutic results in a mouse mannequin of metastatic bone most cancers. To this finish, we established a syngeneic murine bone most cancers ache mannequin by inoculating Lewis lung carcinoma (LLC, 2 × 105 cells in 2 µl) cells into the femora of C57BL/6 mice. Automobile or DMXAA (20 mg/kg) was intraperitoneally (i.p.) injected twice to those mice on day 3 (3d) and day 7 (7d) after tumor implantation. Behavioral assessments together with von Frey testing for mechanical allodynia and acetone response length for chilly allodynia have been carried out on the hindpaw of the tumor-bearing leg at baseline (BL), 7d (earlier than drug injection), 10d, and 14d after LLC inoculation. Flinches and guarding conduct for spontaneous/ongoing ache was evaluated on day 14 submit tumor injection (Fig. 1a), as spontaneous ache behaviors have been occasionally noticed at earlier time factors on this mannequin. DMXAA remedy considerably diminished mechanical allodynia on d7, d10, and d14 (Fig. 1b) and chilly allodynia on d10 and d14 after LLC implantation (Fig. 1c). On d14, DMXAA remedy additionally attenuated measures of spontaneous and ongoing ache (Fig. 1d). No obvious intercourse variations have been noticed, because the therapeutic impact of DMXAA on bone most cancers ache existed in each female and male mice (Supplementary Fig. 1a–d), which is congruent with our earlier report23. As well as, we noticed no variations in physique weight between the vehicle- and DMXAA-treated teams (Fig. 1e), indicating the experimental protocol we used is comparatively secure and with out gross systemic gastrointestinal (GI) toxicity. Notably, survival was not the endpoint of this examine and on condition that animals in late phases of this mannequin skilled extreme ache and useful impairment, all mice have been sacrificed at d17 post-inoculation to take care of affordable well being situations and reduce struggling, as in our current examine30.
a Experimental design to check the antinociceptive results of DMXAA within the LLC mannequin of bone most cancers. b Von Frey testing to find out cancer-induced mechanical allodynia, as assessed by withdrawal threshold (left) or withdrawal frequency (0.16 g stimulus; proper) in mice handled with car or DMXAA (20 mg/kg, i.p.) (n = 11 vehicle-treated mice and n = 9 DMXAA-treated mice) ***P < 0.001. c Evaluation of cancer-induced chilly allodynia after LLC inoculation in mice handled with car or DMXAA (n = 11 vehicle-treated mice and n = 9 DMXAA-treated mice). d Comparability of spontaneous ache as indicated by flinching behaviors (left) or guarding behaviors (proper) in car and DMXAA-treated mice on d14 after tumor implantation (n = 11 vehicle-treated mice and n = 8 DMXAA-treated mice). e Measurement of physique weight in car or DMXAA-treated mice on the indicated timepoints (n = 11 vehicle-treated mice and n = 9 DMXAA-treated mice). f Open discipline testing at d14 post-inoculation to find out distance traveled (m) and imply pace (cm/s) over a 30 min length in car or DMXAA handled mice (n = 8 mice/group). Left: consultant traces. Proper: quantification. g Von Frey testing to measure cancer-induced mechanical allodynia in mice handled with car, ADU-S100 (20 mg/kg, i.p.) or ZA (zoledronic acid; 100 µg/kg, i.p.), n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice, ***P < 0.001. h Measurement of cancer-induced chilly allodynia in mice with indicated remedy on day 7, 10 and 14 after LLC inoculation (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice). i Spontaneous ache as decided by flinching behaviors (left) or guarding behaviors (proper) over a 2-min interval on d14 submit inoculation (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice). j Physique weight measurements in mice with the indicated therapies (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice). ok Open discipline testing, measuring distance traveled (m) and imply pace (cm/s) over a 30 min length in car, ADU-S100, and ZA-treated mice at d14 after tumor inoculation (n = 6 mice/group). Left: consultant traces; proper: quantification. All information displayed signify the imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s post-hoc check (b, c, e, g, h, j); two-tailed Scholar’s t-test (d, f); one-way ANOVA with Bonferroni’s post-hoc check (i, ok). Supply information are supplied as a Supply Knowledge file.
Clinically, a vital comorbidity of bone metastasis in sufferers with superior stage cancers is diminished or misplaced mobility, resulting in useful impairment and diminished high quality of life2,8. To find out whether or not STING activation with DMXAA might enhance locomotor operate, we evaluated the motion exercise within the open discipline check. Importantly, mice handled with DMXAA exhibited higher general distance of motion and elevated pace of motion on d14 after tumor inoculation (Fig. 1f). Thus, systemic remedy with DMXAA considerably improved locomotor operate in mice with bone most cancers.
Bisphosphonates are extensively used for the prevention and remedy of metastatic bone cancer-induced skeletal-related occasions (SREs) by selling apoptosis of bone-resorbing osteoclasts. Zoledronic acid (ZA) is without doubt one of the most potent bisphosphonates31, and has additionally been reported to exhibit antitumor results32. Furthermore, given the restricted translational significance of DMXAA as a result of its specificity for murine STING, we additionally sought to check whether or not ADU-S100 might exert related therapeutic results. Following administration of car, ADU-S100 (20 mg/kg), or ZA (100 µg/kg, a extremely efficient dose with minimal toxicity, as demonstrated in earlier research31,32 at d3 and d10, we discovered that ZA failed to cut back cancer-induced mechanical allodynia when analyzing paw withdrawal threshold, however might cut back withdrawal frequency to low-threshold stimulation (0.16 g Von Frey filament) on d10 submit tumor implantation. ADU-S100 remedy, nonetheless, might considerably attenuate mechanical allodynia in each measures on d10 and d14, with results superior to these of ZA (Fig. 1g). ZA diminished chilly allodynia on d14 after tumor inoculation, whereas ADU-S100 diminished chilly allodynia on each d10 and d14, and this impact was considerably higher within the ADU-S100 group in comparison with the ZA group on d14 (Fig. 1h). As well as, mice handled with ADU-S100 however not ZA exhibited diminished spontaneous and ongoing ache in comparison with vehicle-treated mice (Fig. 1i). We discovered that these doses of ADU-S100 and ZA once more had no impact on general physique weight (Fig. 1j), suggesting they’re comparatively secure and with out gross GI toxicity. To once more assess the potential advantages of ZA and ADU-S100 on operate and mobility, we carried out open discipline testing at d14 on mice handled with car, ADU-S100 and ZA at d3 and d10. Notably, mice within the ADU-S100 remedy group however not the ZA remedy group exhibited elevated general distance of motion and elevated pace of motion in contrast with the car remedy group (Fig. 1k). Taken collectively, ADU-S100 was superior to ZA in lowering cancer-induced ache and enhancing locomotor operate.
STING agonists shield in opposition to cancer-induced bone destruction
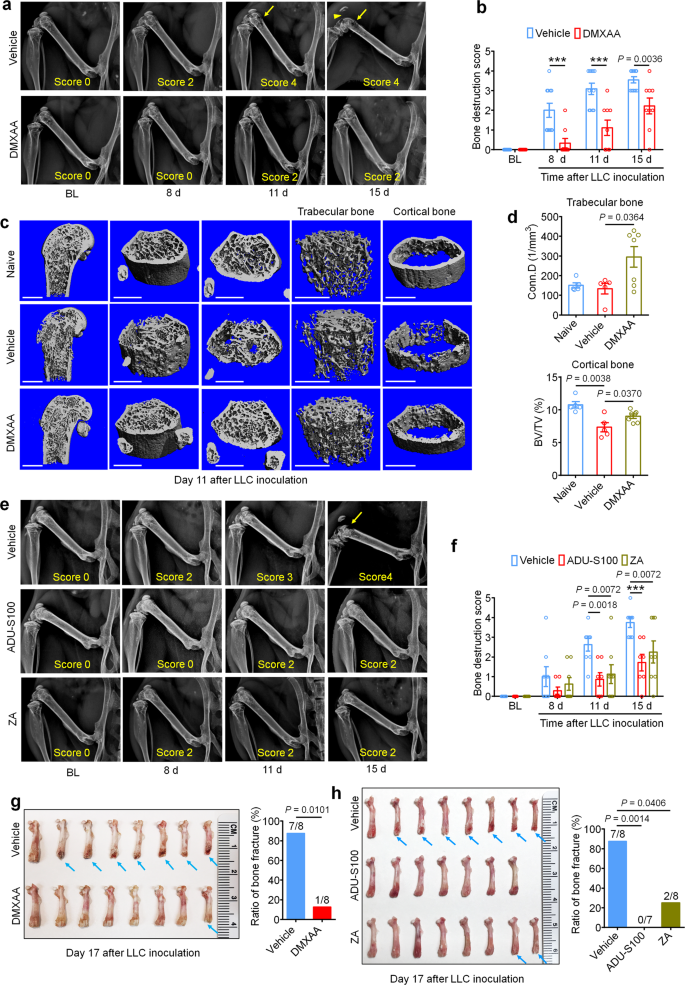
Bone cancer-induced ache normally develops in tandem with the onset of tumor-induced bone destruction. It’s understood that bone most cancers ache is evoked by elements produced instantly by most cancers cells which act on afferent nociceptive nerve fibers within the tumor microenvironment (TME)33,34. As well as, most cancers cells promote bone most cancers ache not directly by accelerating osteoclastogenesis, producing osteoclasts which launch pro-nociceptive elements and promote bone resorption, facilitating bone destruction and painful fractures30,35. Thus, most cancers cells within the bone tumor microenvironment promote bone ache by direct and oblique mechanisms (Supplementary Fig. 1e). In vivo, the LLC cell line is thought to induce osteolytic bone destruction as a result of tumor-induced activation of osteoclast formation and exercise30, recapitulating the pathogenesis of metastatic osteolytic bone cancers in people. Thus, we sought to constantly assess bone destruction utilizing radiography in tumor-bearing mice handled with car or DMXAA (20 mg/kg, i.p. at d3 and d7). The grade of bone destruction was scored on a variety from 0 to five utilizing high-resolution X-ray radiographs of tumor bearing femora, as described by Honore et al.36. On this mannequin, we noticed that LLC inoculation produces a progressively worsening sample of bone destruction which is localized to the distal femur whereas leaving the proximal facet of the femur comparatively unperturbed (Fig. 2a). Furthermore, the tendon connecting the femur and patella is often severed (arrowhead; Fig. 2a). DMXAA remedy decreased the bone destruction rating on d8, d11 and d15 after LLC inoculation in contrast with car group (Fig. 2a, b). No intercourse variations have been noticed within the protecting results of DMXAA on bone destruction (Supplementary Fig. 1f, g). To discover the microarchitecture of bone, we additionally employed micro computed tomography (Micro-CT) with 3-dimentional reconstruction evaluation ex vivo on the distal facet of tumor-bearing femurs. On d11 after tumor implantation and car or DMXAA remedy as in Fig. 2a and 3D reconstruction confirmed much less bone cancer-induced trabecular bone loss and fewer cortical bone lesions in DMXAA-treated mice in contrast with car group (Fig. 2c). Notably, at d11 after LLC inoculation, the lack of cortical bone was most obvious when in comparison with naïve (non-tumor-bearing) mice. Quantitative assessments for bone microstructural parameters demonstrated there may be increased trabecular bone connectivity density (Conn.D) relative to each naïve and LLC-bearing, vehicle-treated mice (Fig. 2nd). As well as, DMXAA rescued LLC-induced cortical bone loss (as assessed by cortical bone quantity/complete quantity (BV/TV; Fig. 2nd). The protecting results of DMXAA on different microstructural parameters of trabecular bone have been much less apparent, though we did observe a major enhance within the variety of trabeculae (Supplementary Figs. 2a–d). Subsequent, we analyzed bone destruction by X-ray radiography following remedy with ADU-S100 or ZA (d3 and d10, as in Fig. 1g). Just like DMXAA, each ADU-S100 and ZA remedy diminished the bone destruction rating at d11 and d15 after tumor inoculation (Fig. 2e, f).
a Consultant radiographs of tumor-bearing femora from car or DMXAA handled mice. Bone destruction rating is indicated in every picture and arrows present bone lesions with scores over 3 whereas arrowhead reveals the indifferent patella. b Quantification for (a) (n = 11 vehicle-treated mice and n = 9 DMXAA-treated mice), ***P < 0.001. c Micro-CT pictures exhibiting trabecular and cortical bone destruction within the distal a part of tumor-bearing femora on d11 after LLC inoculation. Scale bars, 1 mm. d Morphometric quantification of micro-CT pictures with evaluation of trabecular bone (Conn.D; higher) or cortical bone (BV/TV) in tumor-free femora from naïve mice or LLC inoculated femora from car or DMXAA-treated mice (n = 5 naive mice, n = 5 vehicle-treated tumor-bearing mice, and n = 7 DMXAA-treated tumor-bearing mice). e, f Radiographical evaluation of bone destruction in mice administered car, ADU-S100, or ZA on the indicated timepoints after tumor inoculation. e Consultant X-ray pictures. Bone destruction rating is labeled on the underside of every picture and arrow signifies bone destruction rating greater than 3. f Quantification of pictures in e (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice), ***P < 0.001. g Photographs of femurs and quantification of the proportion with bone fracture from tumor bearing femora taken from car or DMXAA-treated mice on d17 after LLC inoculation. Arrows point out the disconnection and absence of the distal a part of the femora (n = 8 mice/group). h Photographs of tumor bearing femora with indicated remedy harvested on d17 after LLC inoculation (left) and quantification of the proportion with bone fracture (proper). Arrows point out lesion and lack of the distal facet of the femora (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice). All information point out the imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s post-hoc check (b, f); two-tailed Scholar’s t-test (d); two-sided Fisher’s precise check (g, h). Supply information are supplied as a Supply Knowledge file.
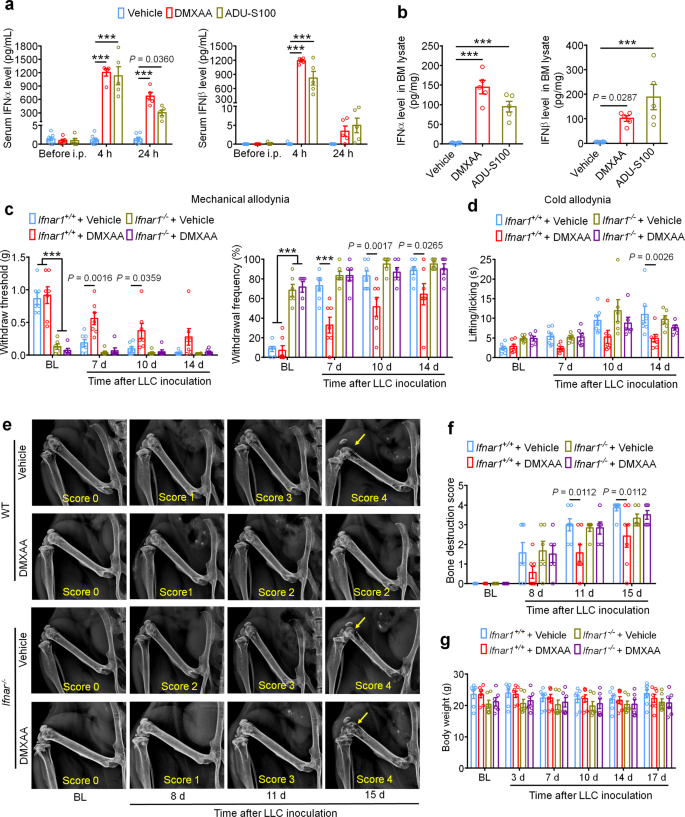
a Evaluation of serum IFN-α (left) and IFN-β (proper) ranges at BL and once more 4 h or 24 h after DMXAA or ADU-S100 remedy on d3 after tumor implantation (n = 7 vehicle-treated mice, n = 5 DMXAA-treated mice, and n = 5 ADU-S100-treated mice), ***P < 0.001. b IFN-α (left) and IFN-β (proper) ranges in bone marrow (BM) lysates from mice handled with car, DMXAA or ADU-S100 (n = 8 vehicle-treated mice, n = 5 DMXAA-treated mice, and n = 5 ADU-S100-treated mice), ***P < 0.001. c von Frey testing to find out withdrawal threshold (left) and frequency (proper) from Ifnar1+/+ or Ifnar1−/− mice handled with car or DMXAA (2 x 20 mg/kg, i.p.). d Evaluation of chilly allodynia in car or DMXAA-treated Ifnar1+/+ mice and Ifnar1−/− mice. e–f Bone destruction scores from radiographs of tumor bearing femora in Ifnar1+/+ and Ifnar1−/− mice with the indicated remedy on d0, d8, d11 and d15 after LLC injection. Arrows present bone lesions with destruction scores over 3. e Consultant X-ray pictures. f Quantification for e. g Physique weight measurement after car or DMXAA remedy. Pattern sizes for c–g have been as follows: n = 7 vehicle-treated Ifnar1+/+ mice, n = 7 DMXAA-treated Ifnar1+/+ mice, n = 6 vehicle-treated Ifnar1−/− mice, n = 6 DMXAA-treated Ifnar1−/− mice. All information point out the imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s post-hoc check (a, c, d, f, g); one-way ANOVA with Bonferroni’s post-hoc check (b). Supply information are supplied as a Supply Knowledge file.
Growth and development of cancer-induced osteolytic bone destruction often results in bone fracture, which is a crucial part of SREs in sufferers with bone metastasis and is related to decreased general survival37. On d17 after LLC inoculation, mice have been euthanized and the tumor bearing femora have been collected and the distal tumor-bearing femur the place bone destruction happens was analyzed. Notably, we discovered that 87.5% (7/8 mice) of vehicle-treated mice suffered bone fractures, whereas solely 12.5% (1/8 mice) mice handled with DMXAA developed distal bone fractures (Fig. 2g). Likewise, 0% (0/7 mice) within the ADU-S100-treated group and 25% (2/8 mice) within the ZA group developed bone fractures (Fig. 2h), indicating each STING agonists and ZA might considerably cut back bone destruction.
STING agonist remedy protects in opposition to breast most cancers induced bone ache and bone destruction
Equally to lung most cancers, breast most cancers can be vulnerable to metastasize to bones and trigger bone destruction8. To discover the potential protecting impact of STING agonists in breast cancer-induced bone destruction, we utilized the E0771 medullary breast carcinoma cell line to ascertain a syngeneic mouse mannequin of breast cancer-induced bone most cancers ache in feminine C57BL/6 mice, as 98% of all breast cancers happen in females38. Just like the LLC line, tumors established with the E0771 line additionally induce osteolytic bone lesions39. After intra-femur inoculation, mice have been handled with car, DMXAA or ADU-S100 adopted by behavioral testing and X-ray radiography of tumor-bearing femurs (Supplementary Fig. 3a). Just like our ends in the LLC bone most cancers ache mannequin, we discovered that DMXAA and ADU-S100 remedy might markedly cut back mechanical allodynia, chilly allodynia and spontaneous ache in comparison with car remedy (Supplementary Fig. 3b–d) however had no impact on physique weight (Supplementary Fig. 3e). Moreover, each DMXAA and ADU-S100 might additionally attenuate bone destruction scored from X-ray pictures of the E0771-bearing femora (Supplementary Fig. 3f, g). Thus, STING agonists can shield in opposition to cancer-induced bone ache and bone destruction brought on by a number of most cancers subtypes vulnerable to bone metastasis.
Protecting impact of DMXAA on bone ache and bone destruction is STING dependent
To confirm the antinociceptive and bone anabolic results of DMXAA are mediated by STING, WT mice and STING “goldenticket” knockout (STINGgt/gt) mice40 have been inoculated with LLC cells intrafemorally adopted by car or DMXAA (20 mg/kg) administration (i.p.) on d3 and d7 submit LLC injection. Notably, STINGgt/gt mice displayed markedly diminished hindpaw withdrawal threshold and elevated withdrawal frequency in von Frey assessments in comparison with WT mice at baseline. DMXAA remedy considerably attenuated mechanical and chilly allodynia in WT mice, and this impact was abolished in STINGgt/gt mice (Supplementary Fig. 4a, b). We additionally measured cancer-induced bone destruction in these mice utilizing radiographic examination of bone destruction of the tumor-bearing distal femurs. We noticed a discount within the bone destruction rating in DMXAA-treated WT mice at d11 and d15 after tumor inoculation, and this impact was abolished in STINGgt/gt mice (Supplementary Fig. 4c, d). We didn’t see physique weight modifications after the experimental manipulations (Supplementary Fig. 4e). Thus, as anticipated, the protecting results of DMXAA on cancer-induced ache and bone destruction are mediated by STING.
IFN-I signaling mediates the protecting results of STING agonists in bone most cancers
STING activation results in the transcriptional induction of interferon response genes and the strong manufacturing and launch of type-I interferons, together with IFN-α and IFN-β. To substantiate that systemic administration of STING agonists results in elevated IFN-I response each systemically and domestically throughout the tumor microenvironment, we analyzed the extent of IFN-α and IFN-β by ELISA. We discovered that serum ranges of IFN-α elevated ~1000-fold 4 h after a single i.p. injection of DMXAA (20 mg/kg) or ADU-S100 (20 mg/kg) on d3 after tumor inoculation in comparison with car group, and this enhance was maintained for as much as 24 h. In the meantime, serum IFN-β ranges have been additionally dramatically upregulated 4 h after DMXAA and ADU-S100 administration (Fig. 3a). On d3 after LLC implantation, the bone marrow (BM) from tumor bearing femora have been additionally collected 4 h after car, DMXAA or ADU-S100 i.p. remedy and analyzed by ELISA. Each IFN-α and IFN-β have been sharply elevated in BM lysate in mice handled with DMXAA or ADU-S100 (Fig. 3b). Thus, systemic administration of STING agonists promoted a strong IFN-I response systemically and within the bone most cancers tumor microenvironment.
The IFN-α/β receptor (IFNAR) is a heterodimeric sign transducing receptor advanced composed of Ifnar1 and Ifnar2, every of which is required for IFN-I signaling. To check how IFN-I signaling contributes to the protecting results of STING agonists within the bone most cancers mannequin, we once more launched LLC cells into the femora of Ifnar1+/+ (WT) or Ifnar1−/− (KO) mice to ascertain the bone most cancers fashions in mice with poor host IFN-I signaling. Just like mice missing STING, we discovered that Ifnar1−/− mice exhibited mechanical hypersensitivity at baseline in comparison with WT littermate management mice (Fig. 3c). Following remedy with car or DMXAA (20 mg/kg, i.p. at d3 and d7), we discovered that DMXAA remedy successfully attenuated cancer-induced mechanical allodynia and chilly allodynia in WT mice however not Ifnar1−/− mice (Fig. 3c, d). On d11 and d15 after tumor inoculation, DMXAA remedy additionally considerably diminished the bone destruction rating with out altering general physique weight in WT mice, however this impact was abolished in Ifnar1−/− mice (Fig. 3e–g). Due to this fact, host IFN-I signaling by Ifnar1 is required for the protecting results of STING agonists on most cancers ache and bone destruction induced by bone most cancers.
DMXAA inhibits bone cancer-induced hyperexcitability of DRG nociceptive neurons
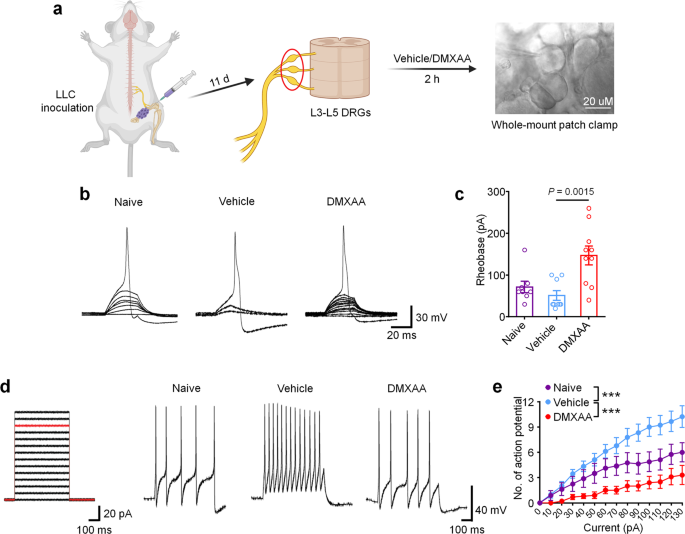
Provided that cancer-induced bone ache in our mannequin is transduced by peripheral nociceptors within the dorsal root ganglion (DRG), we subsequent sought to find out whether or not STING signaling in peripheral sensory neurons contributes to the antinociceptive results of STING agonists in bone most cancers ache. Given our earlier report wherein we demonstrated that STING agonists can instantly suppress nociceptor hyperexcitability in a chemotherapy-induced peripheral neuropathy (CIPN) mannequin of continual ache23, we posited that STING agonists may additionally acutely attenuate bone cancer-induced hyperexcitability of peripheral nociceptors, To check this speculation, WT mice have been inoculated with LLC cells to ascertain bone most cancers fashions and lumbar L3–L5 DRGs have been remoted on d11 and incubated ex vivo with car or DMXAA (30 µM) for two h adopted by patch clamp recordings to measure nociceptor excitability (Fig. 4a). Importantly, on this paradigm, the DRGs from tumor-bearing mice retain their hyperexcitable state relative to DRGs from naïve mice, enabling us to check whether or not DMXAA-mediated STING activation in DRGs can instantly cut back nociceptor excitability. In comparison with car, DMXAA incubation of DRGs markedly elevated the rheobase of nociceptors, a measure of the present required to evoke motion potentials (Fig. 4b, c). As well as, upon examination of current-evoked motion potentials, we discovered that whereas vehicle-treated tumor-bearing mice exhibited nociceptor hyperexcitability relative to naïve (non-tumor-bearing) mice, DMXAA might utterly reverse this cancer-induced nociceptor hyperexcitability (Fig. 4d–e). Though our pattern sizes are comparatively small given the complexity of this ex vivo electrophysiological recording method, the significantly giant magnitude of the consequences enabled us to see statistically vital outcomes. Taken collectively, these information point out that STING activation with DMXAA can suppress cancer-induced hyperexcitability of DRG nociceptors. Provided that DRGs on this preparation are axotomized, thus divorcing them from peripheral or central cell varieties that might contribute to excitability modifications, we conclude that cells current throughout the DRG are enough to mediate these results.
a Schematic of whole-mount DRG preparation, drug remedy, and shiny discipline picture with a recording micropipette sealed on a small-diameter neuron (nociceptor). b Consultant traces of rheobases: present clamp recordings of the membrane potential from small diameter DRG neurons of naïve mice or bone cancer-bearing animals with or with out DMXAA (30 µM, 2 h). Present injection for motion potential induction begins from 0 pA and will increase 10 pA per step for 30 ms. c The averages of rheobases from naïve mice, car or DMXAA-treated group (naïve: n = 8 neurons/4 mice; car: n = 9 neurons/4 mice; DMXAA: n = 10 neurons/4 mice). d Left: injected present steps for the induction of motion potentials begins from 0 pA and will increase 10 pA per step for 300 ms. Proper: consultant traces exhibiting the response to a 110 pA present injection (purple line in left) from small diameter DRG neurons of naïve mice or bone most cancers mice with or with out DMXAA remedy. e Motion potentials in response to growing present amplitude from naïve mice or bone most cancers mice with or with out DMXAA (naïve: n = 8 neurons/4 mice; car: n = 9 neurons/4 mice; DMXAA: n = 10 neurons/4 mice), ***P < 0.001. All information point out the imply ± SEM, one-way ANOVA with Bonferroni’s submit hoc check (c); repeated-measures one-way ANOVA with Bonferroni’s post-hoc check (e). Supply information are supplied as a Supply Knowledge file.
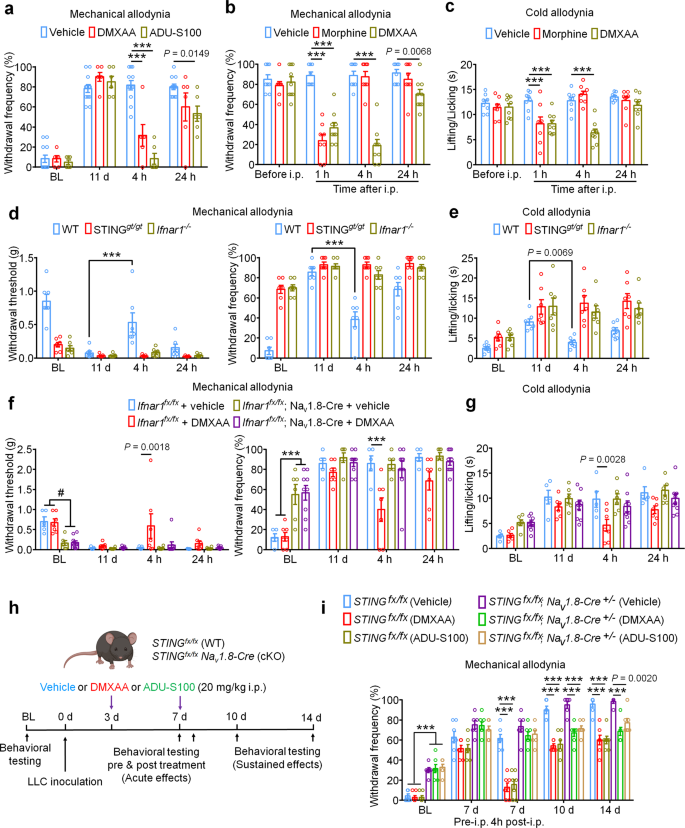
Repeated administration of STING agonists could suppress cancer-induced bone ache by lowering tumor burden, lowering bone destruction, by a neuronal mechanism involving direct suppression of nociceptor exercise, or a mix of all three of those mechanisms. Our electrophysiological information point out that STING agonists can suppress bone cancer-induced ache through a direct neuronal mechanism which is unbiased to results on tumor development or bone destruction. To check whether or not acute administration of STING agonists can suppress bone cancer-induced ache, we carried out behavioral testing in mice on d11 after tumor inoculation 4 h after single i.p. injection of car, DMXAA, or ADU-S100. Notably, each STING agonists induced a considerable discount in mechanical allodynia, chilly allodynia, and spontaneous ache (Fig. 5a–c and Supplementary Fig. 5a, b). Because the pure activators of STING are intracellular double-stranded DNA (dsDNA), through a cGAS-dependent pathway, and through a cGAS-independent pathway involving bacterially-derived cyclic dinucleotides equivalent to 3′3′-cGAMP41, we additionally assessed whether or not dsDNA and three′3′-cGAMP might produce antinociception within the bone most cancers mannequin. Apparently, i.p. administration of dsDNA (30 µg, complexed with LyoVec to facilitate mobile penetration) or cGAMP (20 mg/kg) might attenuate chilly allodynia or/and mechanical allodynia 4 h after injection on d11 submit LLC implantation (Supplementary Fig. 5c–f). Provided that bone most cancers ache is often handled by opioids equivalent to morphine, we examined the analgesic efficiency of DMXAA relative to morphine (10 mg/kg). Notably, whereas each morphine and DMXAA might quickly attenuate mechanical and chilly allodynia with related efficacy 1 h after administration, the consequences of DMXAA have been for much longer lasting (Fig. 5b, c). As well as, we examined whether or not the acute antinociceptive results of DMXAA have been STING- and Ifnar1-dependent by injecting DMXAA (20 mg/kg, i.p.) into WT, STINGgt/gt mice and Ifnar1−/− mice, measuring mechanical and chilly allodynia 4 h after injection. We discovered DMXAA might cut back mechanical allodynia and chilly allodynia in WT mice however not in STINGgt/gt mice or Ifnar1−/− mice (Fig. 5d, e). To additional decide whether or not neuronal IFN-I signaling is accountable for the acute antinociceptive results of STING agonists, we established the bone most cancers ache mannequin utilizing mice missing Ifnar1 selectively in sensory neurons (Ifnar1fx/fx; Nav1.8-Cre; Ifnar1-cKO), or their wildtype littermates. Importantly, we discovered the antinociceptive results conferred by a single administration of DMXAA (20 mg/kg, i.p.) have been current in WT, however not Ifnar1-cKO littermates (Fig. 5f–g). Given the immediacy of those results, and brought along with our electrophysiological information, we conclude that STING agonists exert antinociceptive results through direct actions on nociceptors in an Ifnar1-dependent mechanism.
a Von Frey testing to find out cancer-induced mechanical allodynia, as assessed by withdrawal frequency in mice handled with car (n = 12 mice), DMXAA (20 mg/kg, i.p., n = 6 mice) or ADU-S100 (20 mg/kg, n = 6 mice), ***P < 0.001. Measurement of mechanical allodynia as indicated by paw withdrawal frequency (b) or chilly allodynia (c) 1 h, 4 h or 24 h after a single i.p. injection of car (n = 8 mice), DMXAA (20 mg/kg, n = 9 mice), or morphine (10 mg/kg, n = 8 mice) on d11 after LLC inoculation, ***P < 0.001. d–e Measurement of mechanical allodynia by von Frey testing (d) and chilly allodynia from acetone response (e) after DMXAA i.p. injection in WT, STINGgt/gt or Ifnar1−/− mice (n = 7 mice/group). Measurement of mechanical allodynia by von Frey testing (f) and chilly allodynia by acetone response length (g) after car/DMXAA i.p. injection in Ifnar1fx/fx; Nav1.8-Cre (Ifnar1-cKO) mice (n = 6 mice for car group and n = 9 mice for DMXAA group) or in Ifnar1fx/fx (WT) mice (n = 5 mice for car group and n = 7 mice for DMXAA group), #P = 0.0027 (Ifnar1fx/fx + car vs. Ifnar1fx/fx; Nav1.8-Cre + car); P = 0.0011 (Ifnar1fx/fx + car vs. Ifnar1fx/fx; Nav1.8-Cre + DMXAA); P = 0.0020 (Ifnar1fx/fx + DMXAA vs. Ifnar1fx/fx; Nav1.8-Cre + car); P = 0.0006 (Ifnar1fx/fx + DMXAA vs. Ifnar1fx/fx; Nav1.8-Cre + DMXAA); ***P < 0.001. h–i Measurement of mechanical allodynia by von Frey testing after DMXAA and ADU-S100 remedy (at d3 and d7, i.p.) in STINGfx/fx; Nav1.8-Cre (STING-cKO) mice or STINGfx/fx (WT) mice. h Schematic of experimental design. i Paw withdrawal frequency. n = 6 mice for STINGfx/fx; Nav1.8-Cre (Automobile) group and n = 7 mice for the remainder teams, ***P < 0.001. Notably, DMXAA and ADU-S100 attenuated mechanical allodynia at later time factors (d10, d14) in STING-cKO mice. All information point out the imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s post-hoc check (a–i). Supply information are supplied as a Supply Knowledge file.
To formally check the contribution of STING signaling in peripheral sensory neurons, we utilized mice missing STING selectively in sensory neurons (STINGfx/fx; Nav1.8-Cre; STING-cKO) or their wildtype littermates. Just like STINGgt/gt mice, STING-cKO mice exhibit mechanical hypersensitivity at baseline relative to STING-WT littermate controls, which we beforehand reported23. Following innoculation with LLC cells through intrafemoral injection, STING-WT and STING-cKO mice have been administered car, DMXAA, or ADU-S100 at d3 and d7. Notably, behavioral testing was carried out previous to DMXAA administration at d7 and once more 4 h after administration, permitting us to evaluate the acute results of STING agonist administration (Fig. 5h). Notably, whereas DMXAA and ADU-S100 each considerably attenuated mechanical allodynia 4 h after administration in STING-WT mice, this impact was abolished in STING-cKO mice, suggesting the acute antinociceptive results depend on STING signaling in peripheral sensory neurons. Apparently, nonetheless, the discount in mechanical allodynia at later time factors (d10, d14) was noticed in each STING-WT and STING-cKO mice (Fig. 5i). These information show that the long-term protecting results of STING agonists are depending on their function of antitumor immunity.
STING agonists suppress native bone most cancers tumor burden and additional metastasis
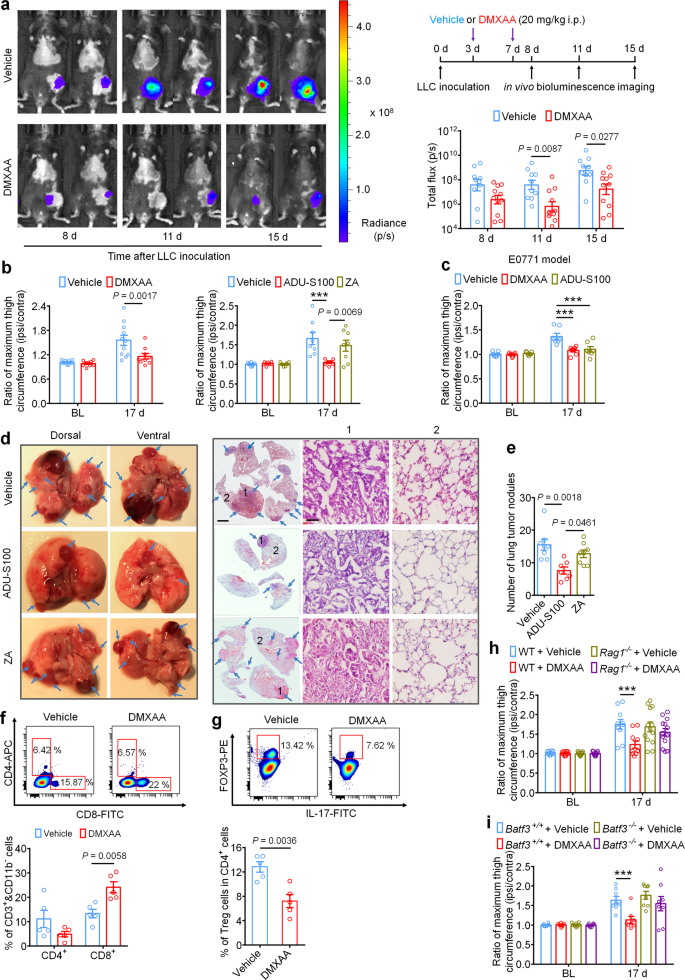
Intratumor injection of STING agonists have been reported to cut back tumor development by selling T cell-mediated antitumor immunity in a number of preclinical animal research12,17,20,42. It’s unknown, nonetheless, whether or not systemic administration of STING agonists can attenuate tumor development within the bone marrow, which is mostly thought to be an overwhelmingly immunosuppressive tumor microenvironment24. To reply this query, luciferase-labeled LLC cells (LL/2-Luc2 cell line) have been used to ascertain the metastatic bone most cancers mannequin through intrafemoral inoculation, thereby enabling measurement of native tumor burden by in vivo bioluminescent imaging. Mice have been handled with car or DMXAA (20 mg/kg, i.p. at d3 and d7), adopted by in vivo bioluminescence imaging at d8, d11, and d15. Notably, mice handled with DMXAA exhibited decrease native tumor burden at d11 and d15, as measured by complete flux of LL/2-Luc2 cells in tumor-bearing mice (Fig. 6a). By d17, tumor development past the conventional anatomic boundaries of the distal femur could possibly be visually noticed, resulting in a rise within the circumference of the tumor-inoculated (ipsilateral) thigh in comparison with the contralateral facet. To quantify this, we measured the ratio of the utmost thigh circumference (ipsilateral/contralateral), which precisely displays native tumor quantity43. Notably, we discovered that DMXAA and ADU-S100 remedy, however not ZA remedy, might cut back the ratio of most thigh circumference in comparison with the vehicle-treated group on d17 in each the LLC and E0771-induced bone most cancers fashions (Fig. 6b, c). To check whether or not these results have been depending on host-intrinsic STING and Ifnar1, we measured native tumor burden utilizing thigh circumference at d17 in STINGgt/gt and Ifnar1−/− mice and located that this protecting impact was abolished in mice missing both STING or Ifnar1 (Supplementary Fig. 6a–c). Thus, systemic administration of STING agonists reduces native bone most cancers tumor burden in a STING- and Ifnar1-dependent method. Nonetheless, the anti-tumor results of DMXAA and ADU-S100 remained in STING-cKO mice with STING knockout in nociceptors (Supplementary Fig. S6d). This consequence means that the long-term protecting results of STING agonists rely on their antitumor immunity.
a In vivo bioluminescence of flux emitted by LL/2-Luc2 carcinoma (LLC) cells in tumor bearing femur after car or DMXAA remedy (2 × 20 mg/kg, i.p.) measured at d8, d11, and d15 submit tumor inoculation (n = 10 vehicle-treated mice, n = 11 DMXAA-treated mice). Photographs (left) have been obtained at 15 min after i.p. injection of D-luciferin (30 mg/kg). Proper, experimental scheme and quantification of a. b Ratio of most thigh circumference reflecting native tumor burden in mice with every indicated remedy on d17 after LLC implantation. Left, car or DMXAA remedy (2 × 20 mg/kg, i.p.; n = 11 vehicle-treated mice and n = 9 DMXAA-treated mice). Proper, car, ADU-S100 (2 × 20 mg/kg, i.p.) or ZA (zoledronic acid; 2 × 100 µg/kg, i.p.) remedy (n = 8 vehicle-treated mice and n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice), ***P < 0.001. c Ratio of most thigh circumference in mice administered with car, DMXAA or ADU-S100 (2 × 100 µg/kg, i.p.) on d17 after implantation of E0771 breast most cancers cells (n = 7 mice/group), ***P < 0.001. d Photographs of lung tumor nodules in mice with every indicated remedy on d17 after LLC inoculation. Left, consultant dorsal and ventral murine lung picture, with arrows exhibiting metastatic tumor nodules. Proper, H&E staining for sections from lung samples in Left. Arrows signifies the areas with tumor cells, scale bar, 2 mm. 1 and a couple of are enlarged pictures exhibiting tumor tissue and peritumoral areas, respectively. Scale bar, 50 µm. Observe that tumor cells have giant and irregular nuclei with lack of the conventional alveolar construction. e Quantification of panel d (n = 8 vehicle-treated mice, n = 7 ADU-S100-treated mice, and n = 8 ZA-treated mice). f–g FACS evaluation of CD4+ and CD8+ T cells (f) or Treg cells (g) throughout the bone marrow tumor microenvironment in mice handled with car or DMXAA (2 × 20 mg/kg, i.p.) on d8 post-LLC inoculation (n = 5 mice/group). The gating technique for this determine is supplied in Supplementary Fig. 7. h Native tumor burden as decided by the ratio of most thigh circumference after car or DMXAA remedy in WT (n = 10 mice for car or DMXAA group) and Rag1−/− mice (n = 13 mice for car or DMXAA group) on d17 after LLC implantation, ***P < 0.001. i Native tumor burden as decided by the ratio of most thigh circumference in Batf3+/+ and Batf3−/− mice with indicated remedy measured on d17 after LLC implantation (n = 8 mice/group), ***P < 0.001. Knowledge point out the imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s submit hoc check (a, b, c, h, i); one-way ANOVA with Bonferroni’s submit hoc check (e); two-tailed Scholar’s t-test (f, g). Supply information are supplied as a Supply Knowledge file.
LLC is a murine lung adenocarcinoma cell line which has affinity to metastasize from the unique injection website to pulmonary lobes and type seen tumor nodules44, enabling use of this phenomenon as a measure of metastasis in our mannequin. To check whether or not systemic administration of ADU-S100 (20 mg/kg i.p.) or ZA (100 µg/kg i.p.) at d3 and d7 might cut back lung metastasis, we analyzed lungs from vehicle-, ADU-S100-, or ZA-treated mice at d17 after intrafemoral LLC inoculation. We discovered that mice receiving ADU-S100 exhibited fewer lung tumor nodules in comparison with mice handled with car or ZA (Fig. 6d–e). Thus, systemic STING activation with ADU-S100 can inhibit each native tumor burden in addition to additional tumor metastasis.
Mechanistically, the antitumor results of STING pathway activation are mainly attributed to antigen presenting cell (APC)-mediated activation of CD8+ T cells45. To check whether or not systemic STING activation can promote CD8+ T-cell infiltration into the immunosuppressive tumor microenvironment of the bone marrow in our bone most cancers mannequin, mice have been administered DMXAA (20 mg/kg i.p.) at d3 and d7 and bone marrow was collected from tumor-bearing femora 24 h after the second DMXAA injection for evaluation of tumor-infiltrating lymphocytes (TILs) by circulate cytometry (gating technique supplied in Supplementary Fig. S7). Importantly, we discovered that DMXAA remedy considerably elevated the proportion of (CD11b− CD3+) CD8+ T cells with out considerably altering the proportion of (CD11b− CD3+) CD4+ T cells within the bone marrow tumor microenvironment (Fig. 6f). We additional analyzed the proportion of immunosuppressive (CD3+ CD4+) Foxp3+, IL-17− regulatory T (Treg)cells and located that DMXAA remedy decreased the proportion of Treg cells within the bone marrow (Fig. 6g). To check whether or not STING agonist-induced discount in tumor burden is because of T cell-mediated antitumor immunity, we launched LLC cells into the intrafemoral cavity of WT or Rag1−/− mice missing mature B and T cells, adopted by car or DMXAA remedy (20 mg/kg i.p. at d3 and d7 post-inoculation) and measurement of most thigh circumference at d17 as in Fig. 6b. We discovered that DMXAA successfully diminished the ratio of most thigh circumference solely in WT mice however not in Rag1−/− mice (Fig. 6h).
Subsequent, on condition that typical kind 1 dendritic cells (cDC1) have been demonstrated to be vital for cross-priming adaptive T cell responses in opposition to tumors by STING-mediated IFN-I induction, we additionally utilized cDC1-deficient Batf3−/− mice to check whether or not the acute and/or long-term protecting results would rely on adaptive antitumor immunity. As anticipated, upon femoral inoculation of Batf3+/+ or Batf3−/− mice with LLC cells, solely tumor-bearing Batf3+/+ mice however not Batf3−/− mice exhibited a lower in native tumor burden at d17 following DMXAA remedy (Fig. 6i). Thus, we conclude that systemic activation of host-intrinsic STING-mediated IFN-I signaling facilitates antitumor immunity by selling TIL entry into the usually immunosuppressive bone marrow TME.
To additional observe the protecting impact of STING agonist on bone most cancers ache, we used a extra physiological mannequin of bone metastasis by administering LLC cells (200,000 in 100 μl) through intra-caudal artery injection (Supplementary Fig. 8a), which was lately reported to ship most cancers cells to the hind limbs and caudal vertebrae with excessive efficacy and trigger bone destruction46. We discovered that repeated DMXAA remedy on day 3, 7, and 11 (Supplementary Fig. 8b) was additionally enough to attenuate bone destruction localized to the caudal vertebrae and diminished the ratio of vertebral fracture at d21 after LLC injection (Supplementary Fig. 8c, d). This verifies that STING agonist might shield most cancers induced bone destruction. Within the intra-caudal artery LLC injection mannequin, which is a typical tumor metastasis mannequin, we additionally discovered that DMXAA can cut back the variety of lung tumor modules (Supplementary Fig. 8e). Moreover, we discovered that acute remedy with DMXAA at d11 after tumor inoculation might potently suppress bone cancer-induced mechanical allodynia (Supplementary Fig. 8f).
STING agonists inhibit cancer-induced osteoclast differentiation through IFN-I signaling
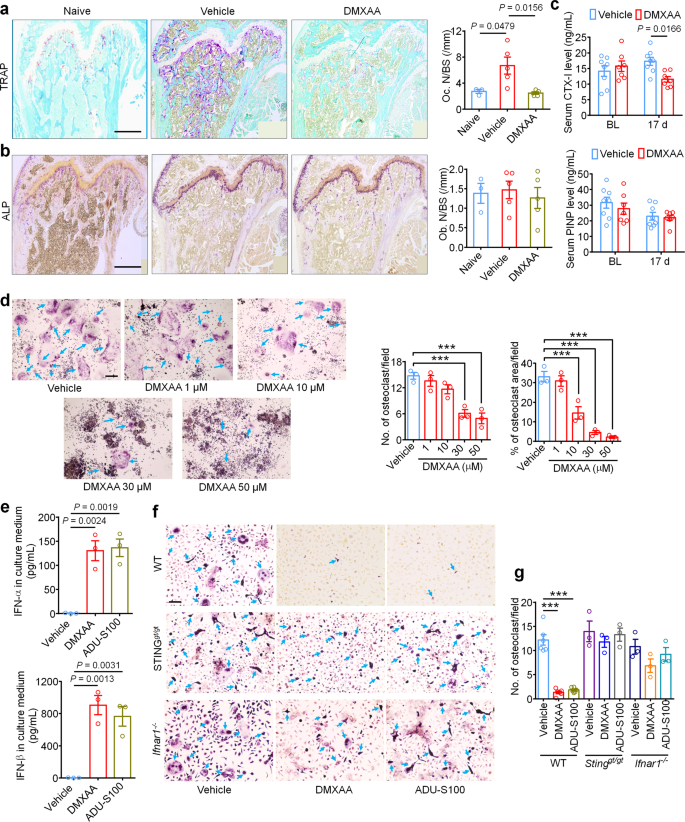
IFN-α and IFN-β have been beforehand reported to inhibit the differentiation of murine and human preosteoclasts into osteoclasts26. Given our information indicating that STING agonists can cut back bone destruction, we sought to find out whether or not the bone protecting results are mediated by direct results on osteoclastogenesis. To this finish, we measured osteoclast cell numbers within the distal tumor-bearing femora at d11 after inoculation in mice handled with car or DMXAA (20 mg/kg i.p. at d3 and d7). Notably, DMXAA-treated mice exhibited far considerably fewer osteoclasts (Fig. 7a), however no modifications have been noticed in bone-forming osteoblasts (Fig. 7b). To additional consider the exercise of osteoclasts and osteoblasts, we collected serum from tumor-bearing mice on BL and d17 after LLC inoculation and measured serum CTX-I and PINP ranges, that are markers for bone resorption and bone formation, respectively30,47. DMXAA might successfully cut back CTX-I ranges on d17 however had no impact on serum PINP ranges (Fig. 7c). These information point out that STING activation with DMXAA can suppress bone cancer-driven osteoclast formation and their bone catabolic exercise.
a Consultant pictures (left) and quantification (proper) of TRAP staining to disclose osteoclast numbers within the tumor-bearing distal femora from mice handled with car or DMXAA (2 × 20 mg/kg, i.p.) measured on d11 after LLC inoculation (n = 3 mice for naïve group and n = 5 mice for car or DMXAA group). Scale bar, 500 µm. b Photographs (left) and quantification (proper) of ALP staining to disclose osteoblasts within the tumor-bearing distal femora at d11 from mice with the indicated therapies (n = 3 mice for naïve group and n = 5 mice for car or DMXAA group). Scale bar, 500 µM. c Measurement of serum CTX-I and PINP ranges by ELISA at BL or d17 in car or DMXAA (2 × 20 mg/kg, i.p.) handled mice (n = 8 vehicle-treated mice and n = 7 DMXAA-treated mice). d TRAP staining revealing osteoclast numbers after differentiation from RAW264.7 cells stimulated with 35 ng/ml RANKL, within the presence of accelerating concentrations of DMXAA. Arrows point out TRAP+ multinucleated osteoclasts. Left, consultant TRAP-stained pictures. Proper, quantification (n = 3 biologically unbiased experimental replicates), ***P < 0.001. Scale bar, 200 µm. e ELISA quantification of IFN-α and IFN-β ranges within the tradition medium of BMDM cells 24 h after DMXAA (30 µM) or ADU-S100 (30 µM) co-incubation. RANKL: 35 ng/ml, MCSF: 20 ng/ml (n = 3 biologically unbiased experimental replicates). f, g TRAP staining for osteoclasts differentiated from BMDM cells from WT mice, STINGgt/gt mice or Ifnar1−/− mice, every handled with car, DMXAA (30 µM) or ADU-S100 (30 µM). RANKL: 35 ng/ml, MCSF: 20 ng/ml. f Consultant pictures of TRAP staining. Arrows point out TRAP+ multinucleated osteoclasts. Scale bar, 100 µm. g Quantification for (f), ***P < 0.001. Pattern sizes for f–g confer with unbiased cultures taken from particular person mice and are as follows: WT/car: n = 6, WT/DMXAA: n = 6, WT/car: n = 6, STINGgt/gt/car: n = 3, STINGgt/gt/DMXAA: n = 3, STINGgt/gt/ADU-S100: n = 3, Ifnar1−/−/car: n = 3, Ifnar1−/−/DMXAA: n = 3, Ifnar1−/−/ADU-S100: n = 3. Knowledge point out the imply ± SEM, one-way ANOVA with Bonferroni’s submit hoc check (a, b, d, e, g); repeated-measures two-way ANOVA with Bonferroni’s post-hoc check (c). Supply information are supplied as a Supply Knowledge file.
Provided that systemic STING agonist remedy reduces osteoclast numbers in vivo, we sought to find out whether or not STING pathway activation can promote osteoclast differentiation in vitro. Murine macrophage RAW 264.7 cells have been handled with RANKL (35 ng/ml, for six days) to advertise osteoclast differentiation30 within the presence of car or an escalating dose of DMXAA or ADU-S100. Importantly, we discovered that each DMXAA and ADU-S100 dose dependently inhibited osteoclast differentiation (Fig. 7d, Supplementary Fig. 9a, b). Bone marrow cells from WT, STINGgt/gt, or Ifnar1−/− mice have been harvested and differentiated into macrophages with 20 ng/ml M-CSF for 3 days. These bone marrow-derived macrophages (BMDM) have been additional induced into osteoclasts with 20 ng/ml M-CSF and 35 ng/ml RANKL for 7 days30. We collected the BMDM tradition medium 24 h after incubation with DMXAA or ADU-S100 and located each agonists induced a drastic enhance in IFN-α and IFN-β ranges within the tradition medium, though IFN-β induction was a lot higher (Fig. 7e). Moreover, TRAP staining confirmed that DMXAA or ADU-S100 remedy (30 µM every) might considerably inhibit osteoclast formation from BMDM from WT mice however not from STINGgt/gt or Ifnar1−/− mice (Fig. 7f, g). To offer additional help that these results have been reliant on IFN-α/β signaling, anti-IFN-α (600 ng/ml) or anti-IFN-β (600 ng/ml) neutralizing antibodies have been added to the induction medium of BMDM adopted by evaluation of osteoclast formation. We discovered that anti-IFN-β antibody might block the inhibition of osteoclast formation by DMXAA or ADU-S100 (Supplementary Fig. 9c–f). Taken collectively, these information point out that activation of the STING/IFN-I signaling axis can inhibit osteoclastogenesis.
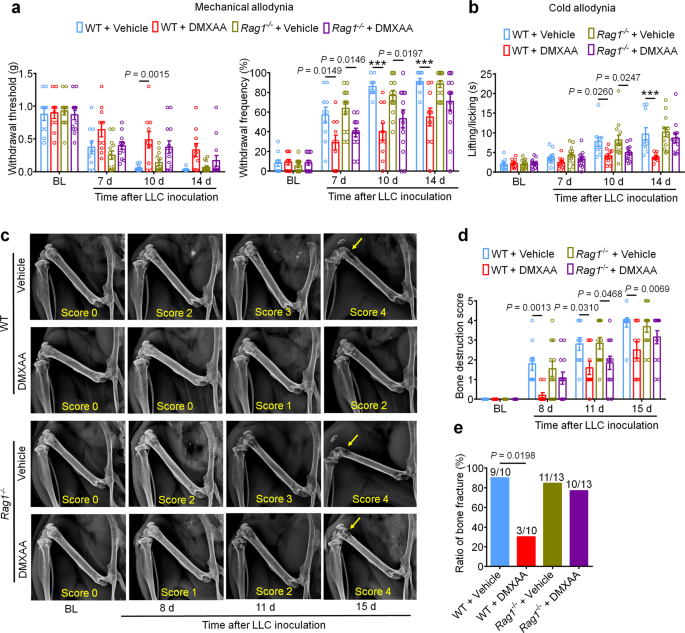
Our findings point out that STING agonists produce antinociception, cut back tumor burden, and cut back bone destruction and osteoclastogenesis. One might argue that each the antinociceptive results and the bone protecting results are secondary to T cell-mediated antitumor immunity. To check this chance, we once more launched LLC cells into the intrafemoral cavity of WT or Rag1−/− mice, adopted by car or DMXAA remedy (20 mg/kg i.p.) at d3 and d7 post-inoculation. Notably, bone cancer-induced mechanical and chilly allodynia have been diminished by DMXAA remedy in each WT and Rag1−/− mice at early phases (d7 and d10), however not at later phases (d14; Fig. 8a, b). Likewise, DMXAA remedy led to an enchancment within the bone destruction rating in each WT and Rag1−/− mice at d11, however not at d15 and at d17 for bone fracture (Fig. 8c–e). Thus, these information point out that DMXAA suppresses ache and bone destruction in a T cell-independent mechanism at early phases, and thus, these results are seemingly as a result of direct suppression of nociceptor excitability and osteoclastogenesis. Because the native tumor burden will increase at later phases, T cell-mediated antitumor immunity could change into important in controlling ache and bone destruction. To additional confirm these findings, we used Batf3+/+ and Batf3−/− mice to ascertain bone most cancers ache mannequin. DMXAA remedy (20 mg/kg i.p., d3 and d7) might attenuate mechanical allodynia or chilly allodynia in Batf3−/− mice on d7 and d10 however not d14 after tumor inoculation (Supplementary Fig. 10a, b). DMXAA additionally diminished bone destruction on d8 and d11 however not d15 in Batf3−/− mice (Supplementary Fig. 10c, d). These information present an extra line of proof that DMXAA suppresses ache and bone destruction in a T cell-independent mechanism at early phases.
a Mechanical allodynia from von Frey check in WT or Rag1−/− mice handled with car or DMXAA (2 × 20 mg/kg, i.p.) on baseline (BL), day 7, 10 and 14 after tumor inoculation. Left, withdrawal threshold. Proper, withdrawal frequency, ***P < 0.001. b Chilly allodynia from acetone check in WT or Rag1−/− mice with indicated remedy, ***P < 0.001. c, d Radiographical evaluation of bone destruction in WT or Rag1−/− mice administered car or DMXAA, measured at BL, d8, d11 and d15 submit LLC inoculation. c Consultant X-ray pictures. Bone destruction rating is labeled on the underside of every picture and arrows point out bone destruction scores of greater than 3. d Quantification for (c). e Quantification of the proportion of mice with distal bone fractures, harvested and analyzed at d17 post-inoculation and with the indicated genotypes and remedy teams. Pattern sizes for a–e have been as follows: n = 10 vehicle-treated WT mice, n = 10 DMXAA-treated WT mice, n = 13 vehicle-treated Rag1−/− mice, and n = 13 DMXAA-treated Rag1−/− mice (pooled from two unbiased experiments). Knowledge are Imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s submit hoc check (a, b, d); two-sided Fisher’s precise check (e). Supply information are supplied as a Supply Knowledge file.
STING agonists inhibit fracture-induced bone ache in tumor-free mice
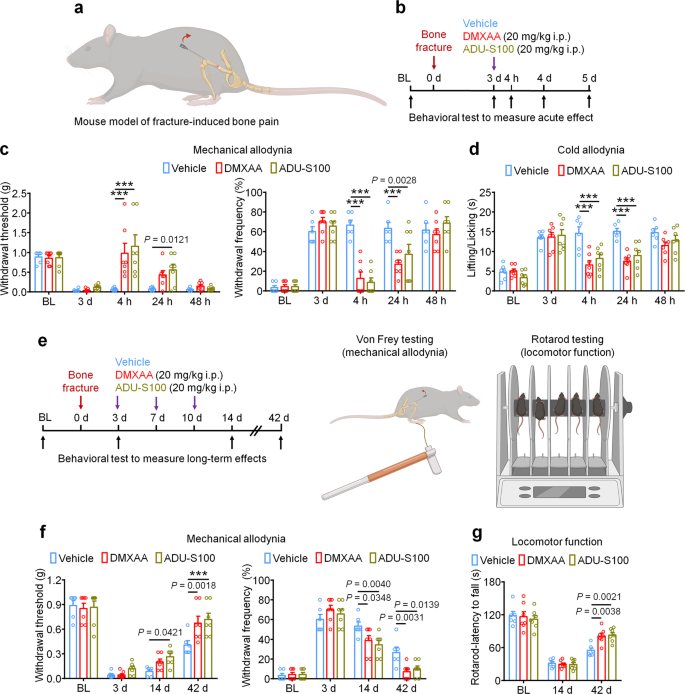
Lastly, we used a bone fracture mannequin which is a tumor-free mannequin of bone ache to analyze the protecting results of STING agonists unbiased of their anti-tumor properties. The mannequin was generated by managed rotation of a rod which is inserted into the femoral cavity of the mouse femur (Fig. 9a). Provided that this mannequin generates intense ache practically instantly following the damage, mice have been handled with car, DMXAA, or ADU-S100 3d (20 mg/kg i.p.) after bone fracture adopted by behavioral testing to measure mechanical and chilly allodynia (Fig. 9b). Apparently, we noticed that each DMXAA and ADU-S100 might potently suppress mechanical and chilly allodynia for as much as 24 h (Fig. 9c, d). To evaluate whether or not repeated administration of STING agonists on this mannequin might facilitate ache decision and useful restoration, mice have been administered car, DMXAA, or ADU-S100 (20 mg/kg i.p.) at d3, d7, and d10 after bone fracture, adopted by measurement of mechanical allodynia and locomotor operate at d14 and d42 (Fig. 9e). Notably, we discovered that DMXAA and ADU-S100 every conferred modest protecting results at d14 and d42 after damage (Fig. 9f) and robustly improved locomotor operate as assessed by the rotarod check (Fig. 9g). Thus, in a cancer-independent mannequin of bone ache, acute administration of STING agonists can attenuate ache transiently, whereas repeated administration of STING agonists can facilitate ache decision and useful restoration following bone fracture.
a Schematic of mouse mannequin of fracture-induced bone ache. b Experimental diagram indicating single remedy of car, DMXAA, or ADU-S100 and behavioral testing. c, d Mechanical allodynia from von Frey check (c) and chilly allodynia from acetone check (d) in mice handled with car, DMXAA, or ADU-S100 (20 mg/kg i.p.) on d3 after bone fracture, ***P < 0.001. e Experimental diagram of repeated administration of car, DMXAA, or ADU-S100 and behavioral testing for the measurement of long-term results. f Von Frey check to find out mechanical allodynia, as assessed by withdrawal threshold (left) or withdrawal frequency (proper) in mice handled with car, DMXAA or ADU-S100 (3 × 20 mg/kg, i.p.) at d14 and d42 after bone fracture, ***P < 0.001. g Locomotor operate of mice handled with car, DMXAA or ADU-S100 (3 × 20 mg/kg, i.p.) at d14 and d42 after damage. Pattern sizes for c, d, f, and g have been as follows: n = 6 vehicle-treated mice, n = 7 DMXAA-treated mice, n = 7 ADU-S100-treated mice. Knowledge are imply ± SEM, repeated-measures two-way ANOVA with Bonferroni’s submit hoc check (c, d, f, g). Supply information are supplied as a Supply Knowledge file.
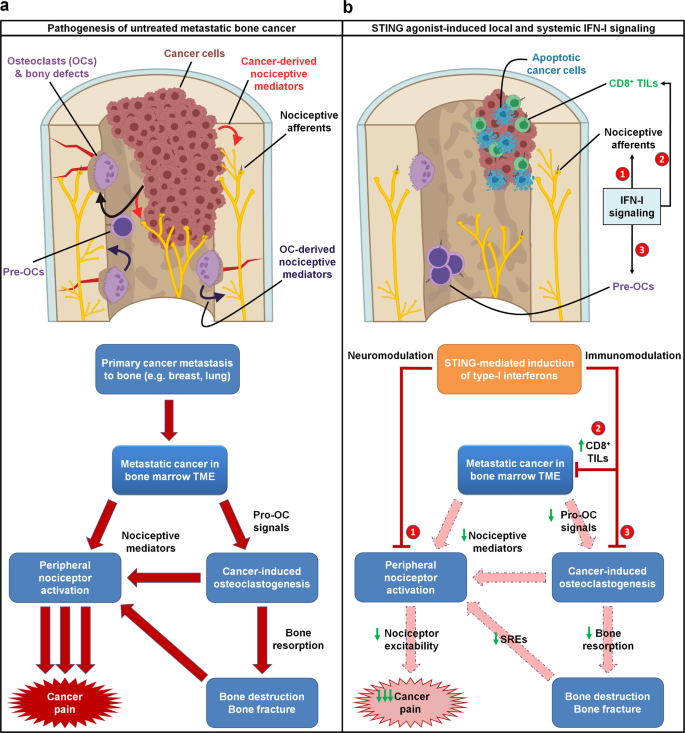
General, we suggest a mechanism by which STING agonists induce strong manufacturing of type-I interferons, which instantly suppress nociceptor excitability and osteoclastogenesis whereas concurrently selling T cell-mediated antitumor immunity. Thus, we posit that STING agonists can acutely suppress most cancers ache by direct results, whereas offering long run aid from bone cancer-induced ache by suppressing osteoclast-mediated bone destruction and relieving native tumor burden (Fig. 10).
a In untreated metastatic bone most cancers, the pathophysiology driving most cancers ache is multifaceted. Metastatic most cancers cells current throughout the bone marrow tumor microenvironment (TME) produce pro-osteoclastogenic alerts, driving cancer-induced osteoclastogenesis and osteoclast overactivation. Each most cancers cells and osteoclasts produce nociceptive mediators, which instantly activate peripheral nociceptive afferents current within the TME to supply ache by a direct mechanism. Moreover, cancer-induced osteoclast overactivation additionally results in elevated bone resorption, resulting in elevated bone destruction and bony fractures, which additionally produce ache by nociceptor activation. b STING agonists dramatically attenuate metastatic bone cancer-associated ache by a number of mechanisms, every mediated by host-intrinsic type-I interferon signaling. First, STING-mediated IFN-I signaling instantly suppresses excitability of peripheral nociceptors (neuromodulation), resulting in acute suppression of ache at some stage in the IFN-I response. As well as, STING-mediated IFN-I signaling promotes CD8+ T cell migration into the bone marrow TME, augmenting antitumor immunity and lowering tumor burden. As well as, IFN-I drives suppression of cancer-induced osteoclastogenesis. These two non-neuronal, immunomodulatory results result in sustained inhibition of ache by (1) lowering most cancers cell- and osteoclast-derived pro-nociceptive mediators and (2) lowering osteoclast-mediated bone resorption, thereby attenuating subsequent bone destruction and bony fractures that often evoke ache and skeletal-related occasions (SRE). Thus, the immunomodulatory and neuromodulatory results of STING agonists every individually suppress ache by actions on totally different cell varieties, and in addition synergistically suppress most cancers ache by a convergence of shared downstream actions. Pre-OCs pre-osteoclasts, TILs tumor infiltrating lymphocytes.